N6-methyladenosine regulates the stability of RNA:DNA hybrids in human cells
- PMID: 31844323
- PMCID: PMC6974403
- DOI: 10.1038/s41588-019-0549-x
N6-methyladenosine regulates the stability of RNA:DNA hybrids in human cells
Abstract
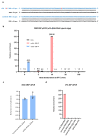
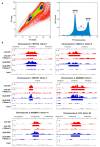
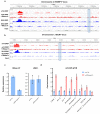
R-loops are nucleic acid structures formed by an RNA:DNA hybrid and unpaired single-stranded DNA that represent a source of genomic instability in mammalian cells1-4. Here we show that N6-methyladenosine (m6A) modification, contributing to different aspects of messenger RNA metabolism5,6, is detectable on the majority of RNA:DNA hybrids in human pluripotent stem cells. We demonstrate that m6A-containing R-loops accumulate during G2/M and are depleted at G0/G1 phases of the cell cycle, and that the m6A reader promoting mRNA degradation, YTHDF2 (ref. 7), interacts with R-loop-enriched loci in dividing cells. Consequently, YTHDF2 knockout leads to increased R-loop levels, cell growth retardation and accumulation of γH2AX, a marker for DNA double-strand breaks, in mammalian cells. Our results suggest that m6A regulates accumulation of R-loops, implying a role for this modification in safeguarding genomic stability.
Conflict of interest statement
Figures


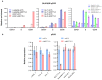



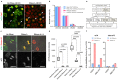

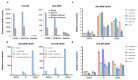


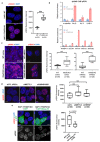
Comment in
-
m6A RNA modification as a new player in R-loop regulation.Nat Genet. 2020 Jan;52(1):27-28. doi: 10.1038/s41588-019-0563-z. Nat Genet. 2020. PMID: 31873295 No abstract available.
References
-
- Santos-Pereira JM, Aguilera A. R loops: new modulators of genome dynamics and function. Nat Rev Genet. 2015;16:583–597. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NC/C013202/1/NC3RS_/National Centre for the Replacement, Refinement and Reduction of Animals in Research/United Kingdom
- BB/M012336/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- NC/K000225/1/NC3RS_/National Centre for the Replacement, Refinement and Reduction of Animals in Research/United Kingdom
- NC/C013105/1/NC3RS_/National Centre for the Replacement, Refinement and Reduction of Animals in Research/United Kingdom
- SP/15/9/31605/BHF_/British Heart Foundation/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- MR/M017354/1/MRC_/Medical Research Council/United Kingdom
- BB/N005759/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 1792340/MRC_/Medical Research Council/United Kingdom
- MR/J007870/1/MRC_/Medical Research Council/United Kingdom
- PG/14/59/31000/BHF_/British Heart Foundation/United Kingdom
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

