Development & Characterization of Fluorescently Tagged Nanocellulose for Nanotoxicological Studies
- PMID: 31844523
- PMCID: PMC6914317
- DOI: 10.1039/C8EN01381K
Development & Characterization of Fluorescently Tagged Nanocellulose for Nanotoxicological Studies
Abstract
The rapid adoption of nanocellulose-based engineered nanomaterials (CNM) by many industries generates environmental health and safety (EHS) concerns. This work presents the development of fluorescently tagged CNM which can be used to study their interactions with biological systems. Specifically, cellulose nano-fibrils and cellulose nano-crystals with covalently attached fluorescein isothiocyanate (FITC) molecules on their surface were synthesized. The fluorescence of the FITC-tagged materials was assessed along with potential FITC detachment under pH conditions encountered in the human gastrointestinal tract, in intracellular compartments, and in cell culture media. Finally, the potential cytotoxicity due to the presence of FITC molecules on the surface of CNM was assessed using a cellular gut epithelium model. The results showed that neither FITC-CNF nor FITC-CNC were cytotoxic and that they have a comparable bioactivity to their untagged counterparts, rendering them suitable for biological studies.
Conflict of interest statement
Conflicts of interest There are no conflicts to declare.
Figures


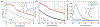
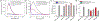
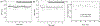

References
-
- Moon RJ, Schueneman GT and Simonsen J, JOM, 2016, 68, 2383–2394.
-
- Ong KJ, Shatkin JA, Nelson K, Ede JD and Retsina T, NanoImpact, 2017, 6, 19–29.
-
- Yu H, Qin Z, Liang B, Liu N, Zhou Z and Chen L, J. Mater. Chem. A, 2013, 1, 3938.
Grants and funding
LinkOut - more resources
Full Text Sources
