Necrotizing enterocolitis is preceded by increased gut bacterial replication, Klebsiella, and fimbriae-encoding bacteria
- PMID: 31844663
- PMCID: PMC6905865
- DOI: 10.1126/sciadv.aax5727
Necrotizing enterocolitis is preceded by increased gut bacterial replication, Klebsiella, and fimbriae-encoding bacteria
Abstract
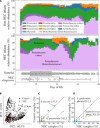
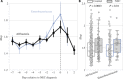
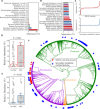
Necrotizing enterocolitis (NEC) is a devastating intestinal disease that occurs primarily in premature infants. We performed genome-resolved metagenomic analysis of 1163 fecal samples from premature infants to identify microbial features predictive of NEC. Features considered include genes, bacterial strain types, eukaryotes, bacteriophages, plasmids, and growth rates. A machine learning classifier found that samples collected before NEC diagnosis harbored significantly more Klebsiella, bacteria encoding fimbriae, and bacteria encoding secondary metabolite gene clusters related to quorum sensing and bacteriocin production. Notably, replication rates of all bacteria, especially Enterobacteriaceae, were significantly higher 2 days before NEC diagnosis. The findings uncover biomarkers that could lead to early detection of NEC and targets for microbiome-based therapeutics.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures



References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

