Gastrointestinal transcription factors drive lineage-specific developmental programs in organ specification and cancer
- PMID: 31844668
- PMCID: PMC6905862
- DOI: 10.1126/sciadv.aax8898
Gastrointestinal transcription factors drive lineage-specific developmental programs in organ specification and cancer
Abstract
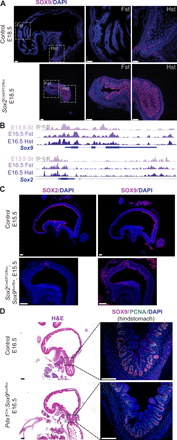
Transcription factors (TFs) are spatially and temporally regulated during gut organ specification. Although accumulating evidence shows aberrant reactivation of developmental programs in cancer, little is known about how TFs drive lineage specification in development and cancer. We first defined gastrointestinal tissue-specific chromatin accessibility and gene expression during development, identifying the dynamic epigenetic regulation of SOX family of TFs. We revealed that Sox2 is not only essential for gastric specification, by maintaining chromatin accessibility at forestomach lineage loci, but also sufficient to promote forestomach/esophageal transformation upon Cdx2 deletion. By comparing our gastrointestinal lineage-specific transcriptome to human gastrointestinal cancer data, we found that stomach and intestinal lineage-specific programs are reactivated in Sox2high /Sox9high and Cdx2high cancers, respectively. By analyzing mice deleted for both Sox2 and Sox9, we revealed their potentially redundant roles in both gastric development and cancer, highlighting the importance of developmental lineage programs reactivated by gastrointestinal TFs in cancer.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures





References
-
- Ferlay J., Shin H.-R., Bray F., Forman D., Mathers C., Parkin D. M., Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 127, 2893–2917 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

