Focusing light inside live tissue using reversibly switchable bacterial phytochrome as a genetically encoded photochromic guide star
- PMID: 31844671
- PMCID: PMC6905864
- DOI: 10.1126/sciadv.aay1211
Focusing light inside live tissue using reversibly switchable bacterial phytochrome as a genetically encoded photochromic guide star
Abstract
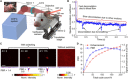
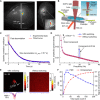
Focusing light deep by engineering wavefronts toward guide stars inside scattering media has potential biomedical applications in imaging, manipulation, stimulation, and therapy. However, the lack of endogenous guide stars in biological tissue hinders its translations to in vivo applications. Here, we use a reversibly switchable bacterial phytochrome protein as a genetically encoded photochromic guide star (GePGS) in living tissue to tag photons at targeted locations, achieving light focusing inside the tissue by wavefront shaping. As bacterial phytochrome-based GePGS absorbs light differently upon far-red and near-infrared illumination, a large dynamic absorption contrast can be created to tag photons inside tissue. By modulating the GePGS at a distinctive frequency, we suppressed the competition between GePGS and tissue motions and formed tight foci inside mouse tumors in vivo and acute mouse brain tissue, thus improving light delivery efficiency and specificity. Spectral multiplexing of GePGS proteins with different colors is an attractive possibility.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures



References
-
- Ntziachristos V., Going deeper than microscopy: The optical imaging frontier in biology. Nat. Methods 7, 603–614 (2010). - PubMed
-
- Sarma R., Yamilov A. G., Petrenko S., Bromberg Y., Cao H., Control of energy density inside a disordered medium by coupling to open or closed channels. Phys. Rev. Lett. 117, 086803 (2016). - PubMed
-
- Popoff S. M., Lerosey G., Carminati R., Fink M., Boccara A. C., Gigan S., Measuring the transmission matrix in optics: An approach to the study and control of light propagation in disordered media. Phys. Rev. Lett. 104, 100601 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

