A randomized controlled phase III study of VB-111 combined with bevacizumab vs bevacizumab monotherapy in patients with recurrent glioblastoma (GLOBE)
- PMID: 31844890
- PMCID: PMC7229248
- DOI: 10.1093/neuonc/noz232
A randomized controlled phase III study of VB-111 combined with bevacizumab vs bevacizumab monotherapy in patients with recurrent glioblastoma (GLOBE)
Abstract
Background: Ofranergene obadenovec (VB-111) is an anticancer viral therapy that demonstrated in a phase II study a survival benefit for patients with recurrent glioblastoma (rGBM) who were primed with VB-111 monotherapy that was continued after progression with concomitant bevacizumab.
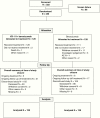
Methods: This pivotal phase III randomized, controlled trial compared the efficacy and safety of upfront combination of VB-111 and bevacizumab versus bevacizumab monotherapy. Patients were randomized 1:1 to receive VB-111 1013 viral particles every 8 weeks in combination with bevacizumab 10 mg/kg every 2 weeks (combination arm) or bevacizumab monotherapy (control arm). The primary endpoint was overall survival (OS), and secondary endpoints were objective response rate (ORR) by Response Assessment in Neuro-Oncology (RANO) criteria and progression-free survival (PFS).
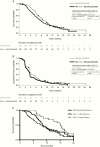
Results: Enrolled were 256 patients at 57 sites. Median exposure to VB-111 was 4 months. The study did not meet its primary or secondary goals. Median OS was 6.8 versus 7.9 months in the combination versus control arm (hazard ratio, 1.20; 95% CI: 0.91-1.59; P = 0.19) and ORR was 27.3% versus 21.9% (P = 0.26). A higher rate of grades 3-5 adverse events was reported in the combination arm (67% vs 40%), mainly attributed to a higher rate of CNS and flu-like/fever events. Trends for improved survival with combination treatment were seen in the subgroup of patients with smaller tumors and in patients who had a posttreatment febrile reaction.
Conclusions: In this study, upfront concomitant administration of VB-111 and bevacizumab failed to improve outcomes in rGBM. Change of treatment regimen, with the lack of VB-111 monotherapy priming, may explain the differences from the favorable phase II results.
Clinical trials registration: NCT02511405.
Keywords: VB-111; anti-angiogenesis; gene therapy; glioblastoma; viral immuno-oncology.
© The Author(s) 2019. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Figures

Comment in
-
Way to Go/No-Go!Neuro Oncol. 2020 May 15;22(5):596-597. doi: 10.1093/neuonc/noaa061. Neuro Oncol. 2020. PMID: 32188987 Free PMC article. No abstract available.
References
-
- Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. - PubMed

