Treatments for preventing recurrence of infection with Pseudomonas aeruginosa in people with cystic fibrosis
- PMID: 31845758
- PMCID: PMC6916140
- DOI: 10.1002/14651858.CD012300.pub2
Treatments for preventing recurrence of infection with Pseudomonas aeruginosa in people with cystic fibrosis
Abstract
Background: Chronic infection with Pseudomonas aeruginosa (PA) in cystic fibrosis (CF) is a source of much morbidity and mortality. Eradication of early PA infection is possible, but can recur in many individuals. We sought to examine strategies to delay the time to PA recurrence in people with CF.
Objectives: To establish whether secondary prevention strategies, using antibiotics or other therapies, increase the chances of people with CF remaining free from PA infection following successful eradication therapy.
Search methods: We searched the Cochrane Cystic Fibrosis Trials Register, compiled from electronic database searches and handsearching of journals and conference abstract books. We also searched ongoing trials registries and the reference lists of relevant articles and reviews. Date of last search: 21 August 2019.
Selection criteria: Randomised controlled trials (and quasi-randomised trials where the risk of bias was low) comparing any treatment modality aimed at preventing recurrence of PA infection with placebo, standard therapy or any other treatment modality in people with CF who have undergone successful eradication of PA.
Data collection and analysis: Two review authors independently assessed trials for inclusion and risk of bias. Quality of the evidence was assessed using GRADE. Conflicts were resolved by discussion and the opinion of a third review author was sought where necessary. Only a subset of participants in the included trial were eligible, therefore individual participant data were requested and obtained from the trial investigators.
Main results: We included one trial (n = 306) in the review; however, only 253 participants had undergone successful eradication of PA, so fulfilling the inclusion criteria for our review. Information presented relates only to the included subset of participants. The trial recruited children aged one to 12 years (mean (standard deviation (SD)) age of 5.8 (3.5) years), 129 participants (51.0%) were female and the median follow-up was 494 days. We compared cycled therapy with tobramycin inhalation solution (TIS), in which participants underwent 28 days of TIS every three months, with culture-based therapy, in which participants were only prescribed medication when a quarterly sputum sample was positive for PA. Reasons for downgrading the quality of the evidence included applicability (only included children), incomplete outcome data and a small number of participants. The time to next isolation of PA was probably shorter with cycled TIS therapy than with culture-based therapy, hazard ratio (HR) 2.04 days (95% confidence interval (CI) 1.28 to 3.26) (moderate-quality evidence). This is in contrast to the main publication of the only included trial, which examined rate of PA positivity rather than time to PA infection and included participants not eligible for inclusion in this review. At the end of the trial, there was no difference between the cycled and culture-based groups in the change from baseline in forced expiratory volume in one second (FEV1) L, mean difference (MD) 0.0 L (95% CI -0.09 to 0.09) or in FEV1 % predicted, MD 0.70% (95% CI -4.33 to 5.73) (both very low-quality evidence). There was no difference in the change from baseline for FVC between the groups. There was also no difference in the frequency of pulmonary exacerbations between groups, MD -0.18 (95% CI -0.51 to 0.14) (moderate-quality evidence). Similarly, there was no difference between groups in the risk of participants developing novel resistant bacteria, RR 1.00 (95% CI 0.67 to 1.5) (moderate-quality evidence). There were more severe adverse events in the cycled group, but the type of treatment probably makes little or no difference to the results, RR 0.65 (95% CI 0.39 to 1.11) (moderate-quality evidence). There was no difference between groups in the change in weight or height from baseline or in rates of adherence to tobramycin or all trial medicines. The included trial did not assess changes in quality of life, the time to chronic infection with PA or the cost-effectiveness of treatment.
Authors' conclusions: Cycled TIS therapy may be beneficial in prolonging the time to recurrence of PA after successful eradication, but further trials are required, specifically addressing this question and in both adults and children.
Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Dr Sally Palser declares that her salary is funded by a Research for Patient Benefit grant from the National Institute of Health Research (NIHR), PB‐PG‐0213‐30055.
Dr Edward Nash declares no known potential conflict of interest.
Dr Arnav Agarwal declares no known potential conflict of interest.
Sherie Smith declares no known potential conflict of interest.
Prof Alan Smyth declares relevant activities of consultancy and an Investigator Initiated Award (independent research grant) from Vertex Pharma. He has given lectures at symposia sponsored by Novartis and TEVA. In addition, Prof Smyth has a patent ALKYL QUINOLONES AS BIOMARKERS OF PSEUDOMONAS AERUGINOSA INFECTION AND USES THEREOF issued.
Figures
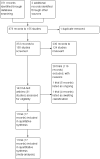






















Update of
References
References to studies included in this review
Treggiari 2011 {published data only}
-
- Anstead M, Lymp J, Khan U, Barbieri J, Langkamp M, Doring G, et al. Pseudomonas aeruginosa serology predicts response to treatment and re‐infection in the EPIC clinical study. Pediatric Pulmonology 2011;46 Suppl 34:303. [Abstract no.: 254; CFGD Register: PI202g]
-
- Anstead M, Saiman L, Mayer‐Hamblett N, Lands LC, Kloster M, Goss CH, et al. Pulmonary exacerbations in CF patients with early lung disease. Journal of Cystic Fibrosis 2014;13(1):74‐9. [CFGD Register: PI202l] - PubMed
-
- Hamblett NM, Retsch‐Bogart GZ, Treggiari M, Kronmal RA, Khan U, Williams J, et al. Safety and efficacy of anti‐pseudomonal therapy for early eradication of Pseudomonas aeruginosa: the EPIC study. Pediatric Pulmonology 2009;44 Suppl 32:183. [CFGD Register: PI202b]
-
- Hamblett NM, Rosenfeld M, Kloster M, Gibson R, Retsch‐Bogart GZ, Thompson V, et al. Impact of successful eradication of pseudomonas aeruginosa on long term outcomes in cystic fibrosis. Pediatric Pulmonology 2014;49(S38):317. [Abstract no.: 283; CENTRAL: CN‐01057040; CFGD Register: PI202q; CRS: 1787622; EMBASE: 71616335]
References to studies excluded from this review
Brett 1992 {published data only}
-
- Brett MM, Simmonds EJ, Ghoneim AT, Littlewood JM. The value of serum IgG titres against Pseudomonas aeruginosa in the management of early pseudomonal infection in cystic fibrosis. Comment in: Arch Dis Child 1993 Mar;68(3):432. Archives of Disease in Childhood 1992;67(9):1086‐8. [CENTRAL: 291223; CFGD Register: PI73; CRS: 5500100000001295] - PMC - PubMed
Carswell 1987 {published data only}
-
- Carswell F, Ward C, Cook DA, Speller DC. A controlled trial of nebulized aminoglycoside and oral flucloxacillin versus placebo in the outpatient management of children with cystic fibrosis. British Journal of Diseases of the Chest 1987;81(4):356‐60. [CENTRAL: 53621; CFGD Register: PI54; CRS: 5500100000000343; PUBMED: 3329531] - PubMed
Connett 2015 {published data only}
-
- Connett GJ, Pike KC, Legg JP, Cathie K, Dewar A, Foote K, et al. Ciprofloxacin during upper respiratory tract infections to reduce Pseudomonas aeruginosa infection in paediatric cystic fibrosis: a pilot study. Therapeutic Advances in Respiratory Disease 2015;9(6):272‐80. [CFGD Register: PI282b] - PubMed
-
- Legg J, Pike K, Cathie K, Dewar A, Foote K, Harris A, et al. A randomized double‐blind, placebo‐controlled trial of ciprofloxacin in the treatment of upper respiratory tract infections in children with cystic fibrosis. Pediatric Pulmonlogy 2014;49 Suppl 38:333. [Abstract no.: 325; CFGD Register: PI282a]
Conway 1985 {published data only}
-
- Conway SP, Miller MG, Ramsden C, Littlewood JM. Intensive treatment of pseudomonas chest infection in cystic fibrosis: a comparison of tobramycin and ticarcillin, and netilmicin and ticarcillin. Acta Paediatrica Scandinavica 1985;74(1):107‐13. [CENTRAL: 37577; CFGD Register: PI34b; CRS: 5500100000000223; PUBMED: 3885674] - PubMed
-
- Conway SP, Miller MG, Ramsden CH, Littlewood JM. Comparison of netilmicin‐plus ticarcillin and tobramycin plus ticarcillin in exacerbations of pseudomonas chest infection in cystic fibrosis. 12th Annual Meeting European Working Group for Cystic Fibrosis; 1983 Oct; Athens, Greece. 1983:271. [CENTRAL: 291257; CFGD Register: PI34a; CRS: 5500100000001322]
Day 1988 {published data only}
-
- Day AJ, Williams J, McKeown C, Bruton A, Weller PH. Evaluation of inhaled colomycin in children with cystic fibrosis. Excerpta Medica, Asia Pacific Congress Series 1988;74:R(c)3. [CENTRAL: 291275; CFGD Register: PI85; CRS: 5500100000001339]
Dinwiddie 1982 {published data only}
-
- Dinwiddie R, Hindmarsh P, Lock P. Azlocillin compared to gentamicin in the treatment of pseudomonas infection in cystic fibrosis. 11th European Cystic Fibrosis Conference; 1982; Brussels. 1982:229. [CENTRAL: 291277; CFGD Register: PI123; CRS: 5500100000001341]
Frederiksen 1997 {published data only}
-
- Frederiksen B, Hansen A, Koch C, Hoiby N. Delay of recurrence of Pseudomonas aeruginosa in patients with cystic fibrosis with inhaled colistin and oral ciproxin: a comparison between 3 weeks and 3 months of treatment. Pediatric Pulmonology 1997; Vol. 23 Suppl 14:288. [Abstract no: 298]
-
- Frederiksen B, Pressler T, Koch C, Hoiby N. Endpoints for evaluating early anti‐pseudomonal treatment: changes in pseudomonas prevalence and in pulmonary function. Pediatric Pulmonology 2003; Vol. 25 Suppl 25:334.
Frederiksen 2006 {published data only}
-
- Frederiksen B, Koch C, Hoiby N, Pressler T, Hansen A. Effect of aerosolised rhDnase (Pulmozyme®) on pulmonary infections in CF: an open randomised study. Pediatric Pulmonology 2000;Suppl 20:246. [CENTRAL: 792917; CFGD Register: BD97a; CRS: 5500100000003555]
-
- Frederiksen B, Pressler T, Hansen A, Koch C, Hoiby N. Effect of aerosolized rhDNase (Pulmozyme) on pulmonary colonization in patients with cystic fibrosis. Acta Paediatrica 2006;95(9):1070‐4. [CENTRAL: 571885; CFGD Register: BD97b; CRS: 5500100000002869; EMBASE: 2006449673; PUBMED: 16938752] - PubMed
Huang 1979 {published data only}
-
- Huang N, Palmer J, Schidlow D, Hsuan F, Hsu C, Goldberg M, et al. Evaluation of antibiotic therapy in patients with cystic fibrosis. Chest 1979;76(3):354‐5. [CENTRAL: 291362; CFGD Register: PI113a; CRS: 5500100000001409]
-
- Huang NN, Palmer J, Braverman S, Keith HH, Schidlow D. Therapeutic efficacy of ticarcillin and carbenicillin in patients with cystic fibrosis: a double blind study. 23rd Annual Meeting Cystic Fibrosis Club Abstracts; 1982 May 14; Washington D.C. 1982:124. [CENTRAL: 291363; CFGD Register: PI113b; CRS: 5500100000001410]
Kenny 2009 {published data only}
-
- Kenny S, Hall V, Goldsmith C, Moore J, Rendall JC, Elborn JS. Eradication of Pseudomonas aeruginosa in adults with CF. Journal of Cystic Fibrosis 2009;8 Suppl 2:S39. [Abstract no.: 158; CFGD Register: PI229]
Knight 1979 {published data only}
-
- Knight RK, Batten JC, Mearns M. A double blind trial of cephalexin in cystic fibrosis patients with pseudomonas in the sputum. 9th Meeting European Working Group for Cystic Fibrosis; 1979 Jun 12‐13; Noordwijkerhout, the Netherlands. 1979:52. [CENTRAL: 291389; CFGD Register: PI124; CRS: 5500100000001431]
Konstan 2011a {published data only}
-
- Chiron R, Geller DE, Angyalosi G, Debonnett L, Yadao A, Bader G, et al. Tobramycin powder for inhalation is effective in advanced stage CF lung disease: the EAGER trial. Journal of Cystic Fibrosis 2014;13 Suppl 2:S57. [Abstract no.: 42; CENTRAL: 996576; CFGD Register: PI239k; CRS: 5500129000000011]
-
- Geller DE, Flume PA, Brockhaus F, Zhang J, Angyalosi G, He E, et al. Treatment convenience and satisfaction of tobramycin inhalation powder (TIP) versus TOBI in cystic fibrosis (CF) patients. Journal of Cystic Fibrosis 2010;9 Suppl 1:S22. [Abstract no.: 82; CENTRAL: 776791; CFGD Register: PI239b; CRS: 5500100000003511]
-
- Geller DE, Flume PA, Konstan M, Angyalosi G, Higgins M. Microbiological and clinical response to tobramycin inhalation powder (TIP™) in cystic fibrosis patients with chronic Pseudomonas aeruginosa (Pa) infection. Journal of Cystic Fibrosis 2011;10 Suppl 1:S21. [Abstract no.: 82; CENTRAL: 848918; CFGD Register: PI239g; CRS: 5500100000010633]
-
- Geller DE, Nasr SZ, Piggott S, He E, Angyalosi G, Higgins M. Tobramycin inhalation powder in cystic fibrosis patients: response by age group. Respiratory Care 2014;59(3):388‐98. [CFGD Register: PI239l; CRS: 5500135000000283; PUBMED: 23983274] - PubMed
-
- Konstan M, Flume PA, Brockhaus F, Angyalosi G, He, E, et al. Safety and efficacy of tobramycin inhalation powder (TIP) in treating CF patients infected with Pseudomonas aeruginosa (Pa). Journal of Cystic Fibrosis 2010;9 Suppl 1:S22. [Abstract no.: 82; CENTRAL: 776166; CFGD Register: PI239a; CRS: 5500100000003510]
Latzin 2008 {published data only}
-
- Latzin P, Fehling M, Bauernfeind A, Reinhardt D, Kappler M, Griese M. Efficacy and safety of intravenous meropenem and tobramycin versus ceftazidime and tobramycin in cystic fibrosis. Journal of Cystic Fibrosis 2008;7(2):142‐6. [CFGD Register: PI209] - PubMed
Loening 1979 {published data only}
-
- Loening Baucke VA, Mischler E, Myers MG. A placebo‐controlled trial of cephalexin therapy in the ambulatory management of patients with cystic fibrosis. Journal of Pediatrics 1979;95(4):630‐7. [CENTRAL: 21139; CFGD Register: PI19b; CRS: 5500100000000098; PUBMED: 383934] - PubMed
-
- Loening‐Baucke VA, Mischler EH, Myers MG. Cephalexin in cystic fibrosis: a placebo‐controlled study. Pediatric Research 1978;12(4 Pt 2):495. [CENTRAL: 189031; CFGD Register: PI19c; CRS: 5500100000000980; EMBASE: 1978335753]
-
- Loening‐Bauke V, Mischler EH, Myers MG. Cephalexin compared to placebo in the management of patients with cystic fibrosis. 19th Cystic Fibrosis Club Abstracts; 1978. 1978:69. [CENTRAL: 291430; CFGD Register: PI19a; CRS: 5500100000001461]
Martin 1980 {published data only}
Murphy 2004 {published data only}
-
- Anbar RD, Yu X, Colin AA. Reduction of pulmonary hospitalizations during a randomized, controlled, open‐label study of tobramycin solution for inhalation in young CF patients with mild lung disease. Pediatric Pulmonology 2003;Suppl 25:296. [CENTRAL: 451881; CFGD Register: PI175b; CRS: 5500100000002383]
-
- Colin AA, Anbar RD, Yu X. Reduction in pulmonary hospitalizations during a randomized, controlled, open‐label study of tobramycin solution for inhalation in young CF patients with mild lung disease. Journal of Cystic Fibrosis 2003;2 Suppl 1:S22. [CENTRAL: 431305; CFGD Register: PI175a; CRS: 5500100000002302]
-
- Murphy TD, Anbar RD, Lester LA, Nasr SZ, Nickerson B, VanDevanter DR, et al. Treatment with tobramycin solution for inhalation reduces hospitalizations in young CF subjects with mild lung disease. Pediatric Pulmonology 2004;38(4):314‐20. [CENTRAL: 496874; CFGD Register: PI175c; CRS: 5500100000002652; EMBASE: 2004402409; PUBMED: 15334509] - PubMed
NCT00645788 {published data only}
-
- Cystic Fibrosis Foundation. Inhaled ciprofloxacin. www.cff.org (www.cff.org/clinicaltrials) (accessed 17 February 2010). [CENTRAL: 744141; CFGD Register: PI261c; CRS: 5500100000003467]
-
- Dorkin H, Criollo M, Reimnitz P, Alder J, Hampel B. Randomized, double‐blind, placebo‐controlled, multicenter study to evaluate the safety and efficacy of inhaled ciprofloxacin compared with placebo in patients with cystic fibrosis‐ a phase IIB study of ciprofloxacin dry powder for inhalation (DPI). Pediatric Pulmonology 2011;46 Suppl 34:296. [Abstract no.: 235; CFGD Register: PI261a]
-
- NCT00645788. Study to evaluate the safety and efficacy of ciprofloxacin (inhaled) in patients with cystic fibrosis [Randomized, double‐blind, placebo‐controlled, multicenter study to evaluate the safety and efficacy of inhaled ciprofloxacin compared to placebo in subjects with cystic fibrosis]. clinicaltrials.gov/show/NCT00645788 (first posted 28 March 2008). [CENTRAL: 744140; CFGD Register: PI261b; CRS: 5500100000003466]
NCT01180634 {published data only}
-
- Flume P, VanDevanter DR, Cohen F, Fleming R, Elborn JS. Safety profile of levofloxacin inhalation solution from 3 controlled cystic fibrosis trials. Journal of Cystic Fibrosis 2015;14 Suppl 1:S87. [Abstract no.: 117; CENTRAL: 1077213; CFGD Register: PI240f // PI283c // PI284c ; CRS: 5500135000001302]
-
- Flume PA, VanDevanter DR, Morgan EE, Dudley MN, Loutit JS, Bell SC, et al. A phase 3, multi‐center, multinational, randomized, double‐blind, placebo‐controlled study to evaluate the efficacy and safety of levofloxacin inhalation solution (APT‐1026) in stable cystic fibrosis patients. Journal of Cystic Fibrosis 2016;15(4):495‐502. [CFGD Register: PI284d; CRS: 5500135000001727; PUBMED: 26852040] - PubMed
-
- Flume PA, VanDevanter DR, Morgan EE, Dudley MN, Loutit JS, Bell SC, et al. A phase 3, multi‐center, multinational, randomized, double‐blind, placebo‐controlled study to evaluate the efficacy and safety of levofloxacin inhalation solution (APT‐1026) in stable cystic fibrosis patients. Journal of Cystic Fibrosis : Official Journal of the European Cystic Fibrosis Society 2016;15(4):495‐502. Online supplement. [CFGD Register: PI284e; CRS: 5500135000001734] - PubMed
-
- NCT01180634. MP‐376 (Aeroquin™, Levofloxacin for Inhalation) in patients with cystic fibrosis [A phase 3, multi‐center, multinational, randomized, double‐blind, placebo‐controlled study to evaluate the efficacy and safety of MP‐376 (Levofloxacin Inhalation Solution; Aeroquin™) in stable cystic fibrosis patients]. clinicaltrials.gov/show/NCT01180634 (first posted 12 August 2010). [CENTRAL: 1012532; CFGD Register: PI284a; CRS: 5500131000000190]
-
- Devanter D, Flume PA, Fleming R, Elborn J. How often is pulmonary exacerbation defined by ≥4 Fuchs criteria associated with antibiotic treatment?. Pediatric Pulmonology 2014;49 Suppl 38:356. [Abstract no.: 388; CENTRAL: 1012531; CFGD Register: PI283b // PI284b ; CRS: 5500131000000188]
Proesmans 2013 {published data only}
-
- NCT01400750. Comparison of 2 treatment regimens for eradication of p aeruginosa infection in children with cystic fibrosis [Prospective randomized trial comparing oral ciproxin plus inhaled colistin with tobramycin for inhalation for eradication of p aeruginosa infection in children with cystic fibrosis]. clinicaltrials.gov/show/NCT01400750 (first received 2011 July 22). [CENTRAL: CN‐01487718; CFGD Register: PI208e; CRS: 8243498]
-
- Proesmans M, Boulanger L, Vermeulen F, Boeck K. Eradication of recent Pseudomonas aeruginosa isolation: TOBI versus colistin/ ciprofloxacin. Journal of Cystic Fibrosis 2008;7 Suppl 2(Suppl 2):S64. [CFGD Register: PI208a]
-
- Proesmans M, Boulanger L, Vermeulen F, Boeck K. Eradication of recent Pseudomonas aeruginosa isolation: TOBI versus colistin/ciprofloxacin. Pediatric Pulmonology 2009;44 Suppl 32(S32):321. [Abstract no.: 311; CFGD Register: PI208b]
-
- Proesmans M, Boulanger L, Vermeulen F, Boeck K. Eradication of recent pseudomonas aeruginosa infection: TOBI versus Colistineb®/ ciprofloxacin. Journal of Cystic Fibrosis 2011;10 Suppl 1:S26. [Abstract no.: 102; CFGD Register: PI208c]
-
- Proesmans M, Vermeulen F, Boulanger L, Verhaegen J, Boeck K. Comparison of two treatment regimens for eradication of Pseudomonas aeruginosa infection in children with cystic fibrosis. Journal of Cystic Fibrosis 2013;12(1):29‐34. [CFGDRegister: PI208d] - PubMed
Ramsey 1999 {published data only}
-
- Birnbaum HG, Greenberg P, Finkelstein S, Berndt E, Otto KL, Montgomery AB, et al. Economic analysis of hospitalization and home IV anti‐pseudomonal antibiotic use in CF patients on tobramycin solution for inhalation (TOBI®). Pediatric Pulmonology 1998;Suppl 17:273. [CENTRAL: 792738; CFGD Register: PI120f; CRS: 5500100000003553]
-
- Bowman CM. The long‐term use of inhaled tobramycin in patients with cystic fibrosis. Journal of Cystic Fibrosis 2002;1 Suppl 2:S194‐8. [CENTRAL: 451888; CFGD Register: PI120cc; CRS: 5500100000002389; PUBMED: 15463834] - PubMed
-
- Burns JL, Dalfsen JM, Shawar RM, Otto KL, Garber RL, Quan JM, et al. Effect of chronic intermittent administration of inhaled tobramycin on respiratory microbial flora in patients with cystic fibrosis. Journal of Infectious Diseases 1999;179(5):1190‐6. [CENTRAL: 161606; CFGD Register: PI120i; CRS: 5500100000000895; PUBMED: 10191222] - PubMed
-
- Casey S, Ramsey B, Borowitz D. Nutritional benefits of chronic intermittent Pseudomonas aeruginosa suppression with tobramycin solution for inhalation in adolescents. 13th International Cystic Fibrosis Congress; 2000 June 4‐8; Stockholm, Sweden. 2000:172. [CENTRAL: 302945; CFGD Register: PI120ff; CRS: 5500100000001685]
-
- Enger C, Rothman K, Kylstra JW. Mortality rates during 2 years of treatment with intermittent inhaled tobramycin (TOBI) in CF. Pediatric Pulmonology 1999;Suppl 19:339‐40. [CENTRAL: 291292; CFGD Register: PI120m; CRS: 5500100000001353]
Ratjen 2010 {published data only}
-
- ISRCTN80955954. Elite study: the microbiological efficacy and safety of two treatment regimens of inhaled tobramycine nebuliser solution (TNS) for the treatment of early onset pseudomonas aeruginosa lower respiratory tract infection in subjects with cystic fibrosis. www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN80955954 (first posted 12 September 2005). [CENTRAL: CN‐01835047; CFGD Register: PI197g; CRS: 10734939]
-
- NTR377. ELITE study [The microbiological efficacy and safety of two treatment regimens of inhaled tobramycine nebuliser solution (TNS) for the treatment of early onset pseudomonas aeruginosa lower respiratory tract infection in subjects with cystic fibrosis. ‐ ELITE]. www.who.int/trialsearch/Trial2.aspx?TrialID=NTR377 (first posted 12 September 2005). [CENTRAL: CN‐01826219; CFGD Register: PI197f; CRS: 10726130]
-
- Ratjen F, Munck A, Campello V. Inhaled tobramycin nebuliser solution for treatment of early Pseudomonas aeruginosa infection: first results from the Elite study. Pediatric Pulmonology 2006;41 Suppl 29:318. [CFGD Register: PI197b]
-
- Ratjen F, Munck A, Campello V. Safety of inhaled tobramycin nebuliser solution for treatment of early pseudomonas aeruginosa infection: first results from the ELITE study. Journal of Cystic Fibrosis 2006;5 Suppl:S22. [CFGD Register: PI197a]
-
- Ratjen F, Munck A, Kho P. Short and long‐term efficacy of inhaled tobramycin in early P. Aeruginosa infection: the ELITE study. Pediatric Pulmonology 2008;43 Suppl 31:319. [CFGD Register: PI197d]
Ratjen 2018 {published data only}
-
- Ratjen F, Alon N, Maykut R, Liu C, Angyalosi G. TOBI® for eradication of early P. aeruginosa infection in paediatric cystic fibrosis patients: the EARLY study. Journal of Cystic Fibrosis 2016;15 Suppl 1:S1. [Abstract no.: WS01.2; CFGD Register: PI290]
-
- Ratjen F, Moeller A, McKinney ML, Asherova I, Alon N, Maykut R, et al. Eradication of early P. aeruginosa infection in children <7years of age with cystic fibrosis: the early study. Journal of Cystic Fibrosis 2019; Vol. 18, issue 1:78‐85. [CFGD Register: PI290b; DOI: 10.1016/j.jcf.2018.04.002] - DOI - PubMed
Schaad 1989 {published data only}
-
- Schaad UB, Wedgwood Krucko J, Guenin K, Buehlmann U, Kraemer R. Antipseudomonal therapy in cystic fibrosis: aztreonam and amikacin versus ceftazidime and amikacin administered intravenously followed by oral ciprofloxacin. European Journal of Clinical Microbiology & Infectious Diseases 1989;8(10):858‐65. [CENTRAL: 64127; CFGD Register: PI63; CRS: 5500100000000416; PUBMED: 2512129] - PubMed
Singh 2013 {published data only}
-
- Singh SB, Shelton AU, Kotek K, Starner TD. A clinically‐embedded trial to evaluate the efficacy of interventions for pre‐pseudomonal pathogens. Pediatric Pulmonology 2013;48 Suppl 36:335. [Abstract no.: 358; CENTRAL: 999884; CFGD Register: PI274; CRS: 5500127000000006]
Taccetti 2012 {published data only}
-
- Cariani L, Defilippi G, Costantini D, Claut L, Clarizia G, D'accico M, et al. Semi‐automated rep‐pcr genotyping of pseudomonas aeruginosa in Italian CF patients in eradication therapy. Pediatric Pulmonology 2010;45(Suppl 33):348. [CFGD Register: PI230c]
-
- Dolce D, Cariani L, Ravenni N, Mergni G, Biffi A, Colombo C, et al. Anti‐ P. aeruginosa antibodies and microbiological outcome in patients treated with early eradication therapy. Pediatric Pulmonology 2013;48(Suppl 36):288. [CFGD Register: PI230i]
-
- EUCTR2008‐006502‐42‐IT. Early antibiotic treatment in pseudomonas aeruginosa eradication in cystic fibrosis patients: a randomised policentric study on two different protocols ‐ #FFC17/2007. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2008‐006502‐42‐IT (first posted 07 April 2010). [CENTRAL: CN‐01871008; CFGD Register: PI230j; CRS: 10770780]
-
- Taccetti G, Bianchini E, Zavataro L, Campana S, Defilippi G, Ravenni N, et al. Pseudomonas aeruginosa eradication in cystic fibrosis: preliminary data from a randomized multicenter study of two different early antibiotic treatment protocols. Pediatric Pulmonology 2010;45 Suppl 33(S33):337. [Abstract no.: 332; CFGD Register: PI230d]
-
- Taccetti G, Bianchini E, Zavataro L, Campana S, Defilippi G, Ravenni N, et al. Pseudomonas aeruginosa microbiological status and emergence of other pathogens after early eradication treatment in cystic fibrosis: a post‐trial follow‐up. Pediatric Pulmonology 2011;46 Suppl 34:317. [Abstract no.: 292; CFGD Register: PI230e]
TORPEDO Trial {published data only}
-
- Cazares A, Figueroa W, Kenna D, Langton‐Hewer S, Smyth A, Winstanley C. Comparative genomics study of a set of Pseudomonas aeruginosa isolates from the TORPEDO‐CF trial. Journal of Cystic Fibrosis 2019;18 Suppl 1:S1. [Abstract no.: WS01‐2; CENTRAL: CN‐01989497; CFGD Register: PI299h; CRS: 12180811; EMBASE: 2001976388]
-
- EUCTR2009‐012575‐10‐SE. Trial of optimal therapy for pseudomonas eradication in cystic fibrosis ‐ TORPEDO‐CF. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2009‐012575‐10‐SE 2016. [CENTRAL: CN‐01798235; CFGD Register: PI299d; CRS: 10698253]
-
- ISRCTN02734162. Trial of optimal therapy for pseudomonas eradication in cystic fibrosis. www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN02734162 (first received 22 May 2009). [CENTRAL: CN‐01807478; CFGD Register: PI299f; CRS: 10707427]
-
- Langton Hewer S. TORPEDO‐CF. www.controlled‐trials.com/ISRCTN02734162/torpedo‐cf (first received 10 October 2011). [CFGD Register: PI299c]
-
- Langton Hewer S, Hickey H, Jones A, Blundell M, Smyth AR, on behalf of the TORPEDO‐CF contributors. TORPEDO‐CF‐completion of recruitment to trial of optimal regimen for eradication of new infection with Pseudomonas aeruginosa. Journal of Cystic Fibrosis 2017;16 Suppl 1:S80. [CFGD Register: PI299a]
Valerius 1991 {published data only}
-
- Valerius NH, Koch C, Hoiby N. Prevention of chronic Pseudomonas aeruginosa colonisation in cystic fibrosis by early treatment. Lancet 1991;338(8769):725‐6. [CENTRAL: 78099; CFGD Register: PI70b; CRS: 5500100000000484; PUBMED: 1679870] - PubMed
-
- Valerius NH, Koch C, Hoiby N. Prevention of chronic colonization with Pseudomonas aeruginosa in patients with CF by early treatment with ciprofloxacin and colistin aerosol inhalations. Pediatric Pulmonology 1990;9 Suppl 5:248. [Abstract no.: 219; CENTRAL: 291624; CFGD Register: PI70a; CRS: 5500100000001626]
Wiesemann 1998 {published data only}
-
- Ratjen F, Steinkamp G, Doring G, Bauernfeind A, Wiesemann HG, Hardt H. Prevention of chronic pseudomonas aeruginosa infection by early inhalation therapy with tobramycin. Pediatric Pulmonology 1994;Suppl 10:255. [CENTRAL: 291534; CFGD Register: PI101a; CRS: 5500100000001553]
-
- Wiesemann HG, Steinkamp G, Ratjen F, Bauernfeind A, Przyklenk B, Doring G, et al. Placebo‐controlled, double‐blind, randomized study of aerosolized tobramycin for early treatment of Pseudomonas aeruginosa colonization in cystic fibrosis. Pediatric Pulmonology 1998;25(2):88‐92. [CENTRAL: 682749; CFGD Register: PI101b; CRS: 5500100000003296; EMBASE: 1998078952; PUBMED: 9516091] - PubMed
References to studies awaiting assessment
OPTIMIZE 2018 {published data only}
-
- Mayer‐Hamblett N, Retsch‐Bogart G, Kloster M, Accurso F, Rosenfeld M, Albers G, et al. Azithromycin for early Pseudomonas infection in cystic fibrosis: the Optimize randomized trial. American Journal of Respiratory and Critical Care Medicine 2018;198(9):1177‐87. [CFGD Register: MA31b; DOI: 10.1164/rccm.201802-0215OC; PUBMED: 29890086] - DOI - PMC - PubMed
-
- Mayer‐Hamblett N, Retsch‐Bogart G, Kloster M, Accurso F, Rosenfeld M, Albers G, et al. Erratum: azithromycin for Early Pseudomonas Infection in Cystic Fibrosis. The OPTIMIZE Randomized Trial [Erratum: Azithromycin for early Pseudomonas infection in cystic fibrosis. The OPTIMIZE randomized trial (American Journal of Respiratory and Critical Care Medicine (2018) 198 (1177–1187) DOI: 10.1164/rccm.201802‐0215OC)]. American Journal of Respiratory and Critical Care Medicine 2019;199(6):809. [CENTRAL: CN‐01967101; CFGD REgister: MA31d; CRS: 11932900; DOI: 10.1164/rccm.201802-0215OC; EMBASE: 2001700512; PUBMED: 30874457] - DOI - PMC - PubMed
-
- NCT02054156. Optimizing treatment for early pseudomonas aeruginosa infection in cystic fibrosis [Optimizing treatment for early pseudomonas aeruginosa infection in cystic fibrosis: the OPTIMIZE multicenter, placebo‐controlled, double‐blind, randomized trial]. clinicaltrials.gov/show/NCT02054156 (first received 2014 February 04). [CENTRAL: CN‐01543461; CFGD Register: MA31c; CRS: 8295539]
-
- Ramsey BW, Retsch‐Bogart GZ, Kloster M, Buckingham R, Hamblett NM. Efficacy and safety of azithromycin for treatment of early pseudomonas in cystic fibrosis: the OPTIMIZE trial. Pediatric Pulmonology 2017;52 Suppl 47:380‐1. [Abstract no. : 434; CFGD Register: MA31a]
References to ongoing studies
Larsson 2011 {published data only}
-
- EUCTR2011‐000801‐39‐SE. Placebo controlled clinical study to evaluate efficacy and safety of an antibody derived from hens’ eggs building a barrier in the respiratory tract against the Pseudomonas germ in order to prevent infection with Pseudomonas in patients suffering from cystic fibrosis [www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2011‐000801‐39‐SE]. (first received 13 December 2011). [CFGD Register: PI252c]
-
- Kollberg H, Larsson A, Nilsson E. Anti‐pseudomonas IGY ready for phase III. Pediatric Pulmonology 2010;45 Suppl 33:343. [Abstract no.: 345; CENTRAL: 848799; CFGD Register: PI252a; CRS: 5500100000003664]
-
- Larsson A. Phase III study (IMPACTT) on anti‐pseudomonas IgY. Journal of Cystic Fibrosis 2011;10 Suppl 1:S24. [Abstract no.: 92; CENTRAL: 848800; CFGD Register: PI252b; CRS: 5500100000003666]
-
- NCT01455675. Efficacy study of igy (antibody against pseudomonas) in cystic fibrosis patients [Phase III study to evaluate clinical efficacy and safety of avian polyclonal anti‐pseudomonas antibodies (igy) in prevention of recurrence of pseudomonas aeruginosa infection in cystic fibrosis patients]. clinicaltrials.gov/show/nct01455675 (first received 2011 October 20). [CENTRAL: CN‐01533433; CFGD Register: PE252e; CRS: 8286130]
-
- Schuster A, Bend J, Hoiby N, Verde PE, Rottmann A, Larsson A, et al. Clinical study to evaluate an anti‐Pseudomonas aeruginosa IgY gargling solution (EUDRACT 2011‐000801‐39). Journal of Cystic Fibrosis 2019;18:S23. [Abstract no.: WS12‐5; CENTRAL: CN‐01990653; CFGD Register: PI252d; CRS: 12181829; EMBASE: 2001976053]
Additional references
Armstrong 1996
-
- Armstrong DS, Grimwood K, Carlin JB, Carzino R, Olinsky A, Phenlan PD. Bronchoalveolar lavage or oropharyngeal cultures to identify lower respiratory pathogens in infants with cystic fibrosis. Pediatric Pulmonology 1996;21(5):267‐75. - PubMed
Barben 2008
-
- Barben J, Schmid J. Dental units as infection sources of Pseudomonas aeruginosa. European Respiratory Journal 2008;32(4):1122‐3. - PubMed
Burns 2001
-
- Burns JL, Gibson RL, McNamara S, Yim D, Emerson J, Rosenfeld M, et al. Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. Journal of Infectious Diseases 2001;183(3):444‐52. - PubMed
Cheng 1996
-
- Cheng K, Smyth RL, Govan JR, Doherty C, Winstanley C, Denning N, et al. Spread of beta‐lactam‐resistant Pseudomonas aeruginosa in a cystic fibrosis clinic. Lancet 1996;348(9028):639‐42. - PubMed
Cheung 1994
-
- Cheung YF, Chan CF, Lee CW, Lau YL. An outbreak of pneumocystis carinii pneumonia in children with malignancy. Journal of Paediatrics and Child Health 1994;30(2):173‐5. - PubMed
Clifton 2008
Collaco 2011
Conway 1996
Cystic Fibrosis Foundation 2015
-
- CF Foundation. About Cystic Fibrosis. www.cff.org/What‐is‐CF/About‐Cystic‐Fibrosis/ (accessed 08 December 2015).
Cystic Fibrosis Foundation 2018
-
- Cystic Fibrosis Foundation [US]. Cystic Fibrosis Foundation Patient Registry 2017 Annual Data Report. www.cff.org/Research/Researcher‐Resources/Patient‐Registry/2017‐Patient‐.... Bethesda, Maryland, (accessed prior to 29 July 2019).
Cystic Fibrosis Trust 2009
-
- UK Cystic Fibrosis Trust. Antibiotic Treatment for Cystic Fibrosis – 3rd edition. Report of the UK Cystic Fibrosis Trust Antibiotic Group. www.cysticfibrosis.org.uk/media/82010/antibiotic‐treatment‐for‐cystic‐fi... (accessed 08 December 2015).
Cystic Fibrosis Trust 2015
-
- Cystic Fibrosis Trust. About CF, frequently asked questions. www.cysticfibrosis.org.uk/about‐cf/frequently‐asked‐questions#na (accessed 08 December 2015).
Cystic Fibrosis Trust 2018
-
- Cystic Fibrosis Trust. UK Cystic Fibrosis Registry Annual Data Report 2017. www.cysticfibrosis.org.uk/the‐work‐we‐do/uk‐cf‐registry/reporting‐and‐re.... London: Cystic Fibrosis Trust, (accessed prior to 27 July 2019).
Deeks 2011
-
- Deeks JJ, Higgins JP, Altman DG on behalf of the CSMG, editor(s). Chapter 9: Analysing data and undertaking meta‐analysis. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Dodge 2007
-
- Dodge JA, Lewis PA, Stanton M, Wilsher J. Cystic fibrosis mortality and survival in the UK: 1947‐2003. European Respiratory Journal 2007;29(3):522‐6. - PubMed
Dwan 2013
Döring 2010
Emerson 2002
-
- Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatric Pulmonology 2002;34(2):91‐100. - PubMed
Gee 2000
Govan 1992
-
- Govan JR, Nelson JW. Microbiology of lung infection in cystic fibrosis. British Medical Bulletin 1992;48(4):912‐30. - PubMed
Heinzel 2002
-
- Heinzl B, Eber E, Oberwaldner B, Haas G, Zach MS. Effects of inhaled gentamicin prophylaxis on acquisition of Pseudomonas aeruginosa in children with cystic fibrosis: a pilot study. Pediatric Pulmonology 2002;33(1):32‐7. - PubMed
Heltshe 2015
Higgins 2011a
-
- Higgins JP, Deeks JJ, editor(s). Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
-
- Higgins JP, Altman DG, Sterne JAC on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
-
- Higgins JP, Deeks JJ, Altman DG on behalf of the Cochrane Statistical Methods Group, editor(s). Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hogardt 2010
Hurley 2014
Høiby 2005
-
- Høiby N, Frederiksen B, Pressler T. Eradication of early Pseudomonas aeruginosa infection. Journal of Cystic Fibrosis 2005;4 Suppl 2:49‐54. - PubMed
Institute for Work and Health 2015
-
- Institute for Work and Health. What researchers mean by Primary, Secondary and Tertiary Prevention. At Work 2015;Spring 2015:2.
Jensen 1997
-
- Jensen ET, Giwercman B, Ojeniyi B, Bangsborg JM, Hansen A, Koch C, et al. Epidemiology of Pseudomonas aeruginosa in cystic fibrosis and the possible role of contamination by dental equipment. Journal of Hospital Infection 1997;36(2):117‐22. - PubMed
Johansen 1992
Jones 2001
-
- Jones AM, Govan JR, Doherty CJ, Dodd ME, Isalska BJ, Stanbridge TN, et al. Spread of a multiresistant strain of Pseudomonas aeruginosa in an adult cystic fibrosis clinic. Lancet 2001;358(9281):557‐8. - PubMed
Jones 2003
Jones 2015
-
- Jones P, Palser SC, Prayle AP, Hurley MN, Smyth AR. Secular trends in Pseudomonas aeruginosa acquisition in the United Kingdom: a registry study. Journal of Cystic Fibrosis 2015;14 Supp 1:S31. [Abstract no.: WS20.1]
Khan 2007
-
- Khan NH, Ishii Y, Kimata‐Kino N, Esaki H, Nishino T, Nishimura M, et al. Isolation of Pseudomonas aeruginosa from open ocean and comparison with freshwater, clinical, and animal isolates. Microbial Ecology 2007;53(2):173‐86. - PubMed
Knibbs 2014
Kollberg 2003
-
- Kollberg H, Carlander D, Olesen H, Wejåker P, Johannesson M, Larsson A. Oral administration of specific yolk antibodies (IgY) may prevent Pseudomonas aeruginosa infections in patients with cystic fibrosis: a phase I feasibility study. Pediatric Pulmonology 2003;35(6):433‐40. - PubMed
Konstan 2007
-
- Konstan MW, Morgan WJ, Butler SM, Pasta DJ, Craib ML, Silva SJ, et al. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. Journal of Pediatrics 2007;151(2):134‐9, 139 e1. - PubMed
Kosorok 2001
-
- Kosorok MR, Zeng L, West SE, Rock MJ, Splaingard ML, Laxova A, et al. Acceleration of lung disease in children with cystic fibrosis after Pseudomonas aeruginosa acquisition. Pediatric Pulmonology 2001;32(4):277‐87. - PubMed
Langton Hewer 2017
Mainz 2015
-
- Mainz JG, Gerber A, Lorenz M, Michl R, Hentschel J, Nader A, et al. Pseudomonas aeruginosa acquisition in cystic fibrosis patients in context of otorhinolaryngological surgery or dentist attendance: case series and discussion of preventive concepts. Case Reports in Infectious Diseases 2015;2015:438517. [DOI: 10.1155/2015/438517] - DOI - PMC - PubMed
Mogayzel 2014
-
- Mogayzel PJ Jr, Naureckas ET, Robinson KA, Brady C, Guill M, Lahiri T, et al. Cystic Fibrosis Foundation pulmonary guideline. pharmacologic approaches to prevention and eradication of initial Pseudomonas aeruginosa infection. Annals of the American Thoracic Society 2014;11(10):1640‐50. [DOI: 10.1513/AnnalsATS.201404-166OC] - DOI - PubMed
MS Excel 2016 [Computer program]
-
- Microsoft Corporation. MS Excel. Washington, USA: Microsoft Corporation, 2016.
Munck 2001
-
- Munck A, Bonacorsi S, Mariani‐Kurkdjian P, Lebourgeois M, Gerardin M, Brahimi N, et al. Genotypic characterization of Pseudomonas aeruginosa strains recovered from patients with cystic fibrosis after initial and subsequent colonization. Pediatric Pulmonology 2001;32(4):288‐92. - PubMed
Nelson 2011
-
- Nelson M, Dockrell D, Edwards S, Angus B, Barton S, Beeching N, et al. British HIV Association and British Infection Association guidelines for the treatment of opportunistic infection in HIV‐seropositive individuals 2011. HIV Medicine 2011;12 Suppl 2:1‐140. [DOI: 10.1111/j.1468-1293.2011.00944_1.x] - DOI - PubMed
Nixon 2001
-
- Nixon GM, Armstrong DS, Carzino R, Carlin JB, Olinsky A, Robertson CF, et al. Clinical outcome after early Pseudomonas aeruginosa infection in cystic fibrosis. Journal of Pediatrics 2001;138(5):699‐704. - PubMed
Palser 2016
Panagea 2005
-
- Panagea S, Winstanley C, Walshaw MJ, Ledson MJ, Hart CA. Environmental contamination with an epidemic strain of Pseudomonas aeruginosa in a Liverpool cystic fibrosis centre, and study of its survival on dry surfaces. Journal of Hospital Infection 2005;59(2):102‐7. - PubMed
Peeters 2016
Pirnay 2005
-
- Pirnay JP, Matthijs S, Colak H, Chablain P, Bilocq F, Eldere J, et al. Global Pseudomonas aeruginosa biodiversity as reflected in a Belgian river. Environmental Microbiology 2005;7(7):969‐80. - PubMed
Pitt 1986
Psoter 2013
-
- Psoter KJ, Roos AJ, Wakefield J, Mayer J, Rosenfeld M. Season is associated with Pseudomonas aeruginosa acquisition in young children with cystic fibrosis. Clinical Microbiology and Infection 2013;19(11):E483‐9. - PubMed
Psoter 2015
Quittner 2009
-
- Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire‐Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest 2009;135(6):1610‐8. - PMC - PubMed
Ramsey 1991
-
- Ramsey BW, Wentz KR, Smith AL, Richardson M, Williams‐Warren J, Hedges DL, et al. Predictive value of oropharyngeal cultures for identifying lower airway bacteria in cystic fibrosis patients. American Review of Respiratiry Disease 1991;144(2):331‐7. - PubMed
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rogers 2010
-
- Rogers GB, Skelton S, Serisier DJ, Gast CJ, Bruce KD. Determining cystic fibrosis‐affected lung microbiology: comparison of spontaneous and serially induced sputum samples by use of terminal restriction fragment length polymorphism profiling. Journal of Clinical Microbiology 2010;48(1):78‐86. - PMC - PubMed
Ronchetti 2018
-
- Ronchetti K, Tame JD, Paisey C, Thia LP, Doull I, Howe R, et al. The CF‐Sputum Induction Trial (CF‐SpIT) to assess lower airway bacterial sampling in young children with cystic fibrosis: a prospective internally controlled interventional trial. Lancet Respiratory Medicine 2018;6(6):461‐71. - PMC - PubMed
Rosenfeld 1999
-
- Rosenfeld M, Emerson J, Accurso F, Armstrong D, Castile R, Grimwood K, et al. Diagnostic accuracy of oropharyngeal cultures in infants and young children with cystic fibrosis. Pediatric Pulmonology 1999;28(5):321‐8. - PubMed
Saiman 2014
-
- Saiman L, Siegel JD, LiPuma JJ, Brown RF, Bryson EA, Chambers MJ, et al. Infection prevention and control guideline for cystic fibrosis: 2013 update. Infection Control & Hospital Epidemiology 2014;35 Suppl 1:S1‐S67. - PubMed
Schelstraete 2008
-
- Schelstraete P, Daele S, Boeck K, Proesmans M, Lebecque P, Leclercq‐Foucart J, et al. Pseudomonas aeruginosa in the home environment of newly infected cystic fibrosis patients. European Respiratory Journal 2008;31(4):822‐9. - PubMed
Schelstraete 2010
-
- Schelstraete P, Deschaght P, Simaey L, Daele S, Haerynck F, Vaneechoutte M, et al. Genotype based evaluation of Pseudomonas aeruginosa eradication treatment success in cystic fibrosis patients. Journal of Cystic Fibrosis 2010;9(2):99‐103. - PubMed
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al on behalf of the Cochrane Applicability and Recommendations Methods Group and the Cochrane Statistical Methods Group. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Smyth 2017
-
- Smyth A. The Core Outcome Set for CF (COST‐CF). www.comet‐initiative.org/studies/details/882?result=true. COMET Initiative, (accessed prior to 29 July 2019).
Speert 1987
-
- Speert DP, Campbell ME. Hospital epidemiology of Pseudomonas aeruginosa from patients with cystic fibrosis. Journal of Hospital Infection 1987;9(1):11‐21. - PubMed
Speert 1990
Speert 2002
-
- Speert DP, Campbell ME, Henry DA, Milner R, Taha F, Gravelle A, et al. Epidemiology of Pseudomonas aeruginosa in cystic fibrosis in British Columbia, Canada. American Journal of Respiratory and Critical Care Medicine 2002;166(7):988‐93. - PubMed
Stata 2019 [Computer program]
-
- StataCorp LLC. Stata Statistical Software: Release 16. Version 16. College Station, Texas, USA: StataCorp LLC, 2019.
Sterne 2011
-
- Sterne JA, Egger M, Moher D on behalf of the Cochrane Bias Methods Group, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editor(s). Cochrane Handbook forSystematic Reviews of Interventions Version 5.1.0 updated March 2011. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

