NanoMEA: A Tool for High-Throughput, Electrophysiological Phenotyping of Patterned Excitable Cells
- PMID: 31845810
- PMCID: PMC7547911
- DOI: 10.1021/acs.nanolett.9b04152
NanoMEA: A Tool for High-Throughput, Electrophysiological Phenotyping of Patterned Excitable Cells
Abstract
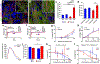
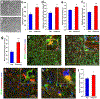
Matrix nanotopographical cues are known to regulate the structure and function of somatic cells derived from human pluripotent stem cell (hPSC) sources. High-throughput electrophysiological analysis of excitable cells derived from hPSCs is possible via multielectrode arrays (MEAs) but conventional MEA platforms use flat substrates and do not reproduce physiologically relevant tissue-specific architecture. To address this issue, we developed a high-throughput nanotopographically patterned multielectrode array (nanoMEA) by integrating conductive, ion-permeable, nanotopographic patterns with 48-well MEA plates, and investigated the effect of substrate-mediated cytoskeletal organization on hPSC-derived cardiomyocyte and neuronal function at scale. Using our nanoMEA platform, we found patterned hPSC-derived cardiac monolayers exhibit both enhanced structural organization and greater sensitivity to treatment with calcium blocking or conduction inhibiting compounds when subjected to high-throughput dose-response studies. Similarly, hPSC-derived neurons grown on nanoMEA substrates exhibit faster migration and neurite outgrowth speeds, greater colocalization of pre- and postsynaptic markers, and enhanced cell-cell communication only revealed through examination of data sets derived from multiple technical replicates. The presented data highlight the nanoMEA as a new tool to facilitate high-throughput, electrophysiological analysis of ordered cardiac and neuronal monolayers, which can have important implications for preclinical analysis of excitable cell function.
Keywords: Multielectrode arrays; cardiomyocyte; electrophysiology; iPSC; nanotopography; neuron.
Conflict of interest statement
Conflict of Interest Statement
Alec S.T. Smith is a scientific advisor for NanoSurface Biomedical and holds uncompensated stock in the company. Deok-Ho Kim is an advisor and co-founder of NanoSurface Biomedical and holds uncompensated stock in the company. Kevin Gray and Jesse Macadangdang are employees at NanoSurface Biomedical.
Figures

References
-
- Hyun SW; Kim BR; Hyun SA; Seo JW J Pharmacol Toxicol Methods 2017, 87, 93–98. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

