CD19-targeting CAR T cell immunotherapy outcomes correlate with genomic modification by vector integration
- PMID: 31845905
- PMCID: PMC6994131
- DOI: 10.1172/JCI130144
CD19-targeting CAR T cell immunotherapy outcomes correlate with genomic modification by vector integration
Abstract
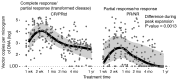
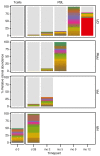
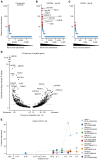
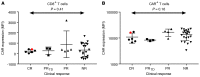
Chimeric antigen receptor-engineered T cells targeting CD19 (CART19) provide an effective treatment for pediatric acute lymphoblastic leukemia but are less effective for chronic lymphocytic leukemia (CLL), focusing attention on improving efficacy. CART19 harbor an engineered receptor, which is delivered through lentiviral vector integration, thereby marking cell lineages and modifying the cellular genome by insertional mutagenesis. We recently reported that vector integration within the host TET2 gene was associated with CLL remission. Here, we investigated clonal population structure and therapeutic outcomes in another 39 patients by high-throughput sequencing of vector-integration sites. Genes at integration sites enriched in responders were commonly found in cell-signaling and chromatin modification pathways, suggesting that insertional mutagenesis in these genes promoted therapeutic T cell proliferation. We also developed a multivariate model based on integration-site distributions and found that data from preinfusion products forecasted response in CLL successfully in discovery and validation cohorts and, in day 28 samples, reported responders to CLL therapy with high accuracy. These data clarify how insertional mutagenesis can modulate cell proliferation in CART19 therapy and how data on integration-site distributions can be linked to treatment outcomes.
Keywords: Immunotherapy; Microbiology.
Conflict of interest statement
Figures

Similar articles
-
CAR T Cell Therapy in Acute Lymphoblastic Leukemia and Potential for Chronic Lymphocytic Leukemia.Curr Treat Options Oncol. 2016 Jun;17(6):28. doi: 10.1007/s11864-016-0406-4. Curr Treat Options Oncol. 2016. PMID: 27098534 Review.
-
Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells.Nature. 2018 Jun;558(7709):307-312. doi: 10.1038/s41586-018-0178-z. Epub 2018 May 30. Nature. 2018. PMID: 29849141 Free PMC article.
-
Ibrutinib for improved chimeric antigen receptor T-cell production for chronic lymphocytic leukemia patients.Int J Cancer. 2021 Jan 15;148(2):419-428. doi: 10.1002/ijc.33212. Epub 2020 Jul 28. Int J Cancer. 2021. PMID: 32683672
-
CD19-Chimeric Antigen Receptor T Cells for Treatment of Chronic Lymphocytic Leukaemia and Acute Lymphoblastic Leukaemia.Scand J Immunol. 2015 Oct;82(4):307-19. doi: 10.1111/sji.12331. Scand J Immunol. 2015. PMID: 26099639 Review.
-
Durable Molecular Remissions in Chronic Lymphocytic Leukemia Treated With CD19-Specific Chimeric Antigen Receptor-Modified T Cells After Failure of Ibrutinib.J Clin Oncol. 2017 Sep 10;35(26):3010-3020. doi: 10.1200/JCO.2017.72.8519. Epub 2017 Jul 17. J Clin Oncol. 2017. PMID: 28715249 Free PMC article. Clinical Trial.
Cited by
-
The Chimeric Antigen Receptor Detection Toolkit.Front Immunol. 2020 Aug 11;11:1770. doi: 10.3389/fimmu.2020.01770. eCollection 2020. Front Immunol. 2020. PMID: 32849635 Free PMC article. Review.
-
CAR T-cell Therapy Meets Clonal Hematopoiesis.Blood Cancer Discov. 2022 Sep 6;3(5):382-384. doi: 10.1158/2643-3230.BCD-22-0067. Blood Cancer Discov. 2022. PMID: 35896010 Free PMC article.
-
Retroviral Insertional Mutagenesis in Humans: Evidence for Four Genetic Mechanisms Promoting Expansion of Cell Clones.Mol Ther. 2020 Feb 5;28(2):352-356. doi: 10.1016/j.ymthe.2019.12.009. Epub 2020 Jan 7. Mol Ther. 2020. PMID: 31951833 Free PMC article. Review.
-
Clinical and Translational Landscape of Viral Gene Therapies.Cells. 2024 Nov 19;13(22):1916. doi: 10.3390/cells13221916. Cells. 2024. PMID: 39594663 Free PMC article. Review.
-
Somatic mutations associate with clonal expansion of CD8+ T cells.Sci Adv. 2024 Jun 7;10(23):eadj0787. doi: 10.1126/sciadv.adj0787. Epub 2024 Jun 7. Sci Adv. 2024. PMID: 38848368 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

