Graft-versus-host disease of the CNS is mediated by TNF upregulation in microglia
- PMID: 31846439
- PMCID: PMC7269577
- DOI: 10.1172/JCI130272
Graft-versus-host disease of the CNS is mediated by TNF upregulation in microglia
Abstract
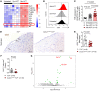
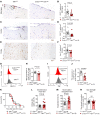
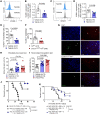
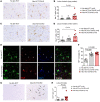
Acute graft-versus-host disease (GVHD) can affect the central nervous system (CNS). The role of microglia in CNS-GVHD remains undefined. In agreement with microglia activation, we found that profound morphological changes and MHC-II and CD80 upregulation occurred upon GVHD induction. RNA sequencing-based analysis of purified microglia obtained from mice with CNS-GVHD revealed TNF upregulation. Selective TNF gene deletion in microglia of Cx3cr1creER Tnffl/- mice reduced MHC-II expression and decreased CNS T cell infiltrates and VCAM-1+ endothelial cells. GVHD increased microglia TGF-β-activated kinase-1 (TAK1) activation and NF-κB/p38 MAPK signaling. Selective Tak1 deletion in microglia using Cx3cr1creER Tak1fl/fl mice resulted in reduced TNF production and microglial MHC-II and improved neurocognitive activity. Pharmacological TAK1 inhibition reduced TNF production and MHC-II expression by microglia, Th1 and Th17 T cell infiltrates, and VCAM-1+ endothelial cells and improved neurocognitive activity, without blocking graft-versus-leukemia effects. Consistent with these findings in mice, we observed increased activation and TNF production of microglia in the CNS of GVHD patients. In summary, we prove a role for microglia in CNS-GVHD, identify the TAK1/TNF/MHC-II axis as a mediator of CNS-GVHD, and provide a TAK1 inhibitor-based approach against GVHD-induced neurotoxicity.
Keywords: Stem cell transplantation; Transplantation.
Conflict of interest statement
Figures




References
-
- Unger ER, Sung JH, Manivel JC, Chenggis ML, Blazar BR, Krivit W. Male donor-derived cells in the brains of female sex-mismatched bone marrow transplant recipients: a Y-chromosome specific in situ hybridization study. J Neuropathol Exp Neurol. 1993;52(5):460–470. doi: 10.1097/00005072-199309000-00004. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

