High-glucose diets induce mitochondrial dysfunction in Caenorhabditis elegans
- PMID: 31846489
- PMCID: PMC6917275
- DOI: 10.1371/journal.pone.0226652
High-glucose diets induce mitochondrial dysfunction in Caenorhabditis elegans
Abstract
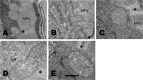
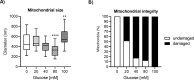
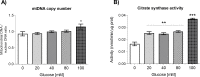
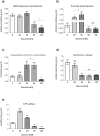
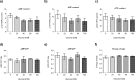
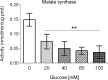
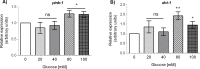
Glucose is an important nutrient that dictates the development, fertility and lifespan of all organisms. In humans, a deficit in its homeostatic control might lead to hyperglucemia and the development of obesity and type 2 diabetes, which show a decreased ability to respond to and metabolize glucose. Previously, we have reported that high-glucose diets (HGD) induce alterations in triglyceride content, body size, progeny, and the mRNA accumulation of key regulators of carbohydrate and lipid metabolism, and longevity in Caenorhabditis elegans (PLoS ONE 13(7): e0199888). Herein, we show that increasing amounts of glucose in the diet induce the swelling of both mitochondria in germ and muscle cells. Additionally, HGD alter the enzymatic activities of the different respiratory complexes in an intricate pattern. Finally, we observed a downregulation of ceramide synthases (hyl-1 and hyl-2) and antioxidant genes (gcs-1 and gst-4), while mitophagy genes (pink-1 and dct-1) were upregulated, probably as part of a mitohormetic mechanism in response to glucose toxicity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

