A Convergent Total Synthesis of the Death Cap Toxin α-Amanitin
- PMID: 31846557
- PMCID: PMC7154671
- DOI: 10.1002/anie.201914620
A Convergent Total Synthesis of the Death Cap Toxin α-Amanitin
Abstract
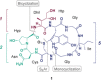
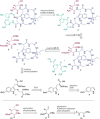
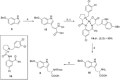
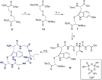
The toxic bicyclic octapeptide α-amanitin is mostly found in different species of the mushroom genus Amanita, with the death cap (Amanita phalloides) as one of the most prominent members. Due to its high selective inhibition of RNA polymerase II, which is directly linked to its high toxicity, particularly to hepatocytes, α-amanitin received an increased attention as a toxin-component of antibody-drug conjugates (ADC) in cancer research. Furthermore, the isolation of α-amanitin from mushrooms as the sole source severely restricts compound supply as well as further investigations, as structure-activity relationship (SAR) studies. Based on a straightforward access to the non-proteinogenic amino acid dihydroxyisoleucine, we herein present a robust total synthesis of α-amanitin providing options for production at larger scale as well as future structural diversifications.
Keywords: amatoxins; asymmetric synthesis; total synthesis; tryptathionine; α-amanitin.
© 2019 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Synthesis of the Death-Cap Mushroom Toxin α-Amanitin.J Am Chem Soc. 2018 May 30;140(21):6513-6517. doi: 10.1021/jacs.7b12698. Epub 2018 Mar 21. J Am Chem Soc. 2018. PMID: 29561592
-
Design, Synthesis, and Biochemical Evaluation of Alpha-Amanitin Derivatives Containing Analogs of the trans-Hydroxyproline Residue for Potential Use in Antibody-Drug Conjugates.Chemistry. 2021 Jul 16;27(40):10282-10292. doi: 10.1002/chem.202101373. Epub 2021 Jun 18. Chemistry. 2021. PMID: 34058032
-
Total Synthesis of α- and β-Amanitin.Angew Chem Int Ed Engl. 2020 Jul 6;59(28):11390-11393. doi: 10.1002/anie.201914935. Epub 2020 Apr 28. Angew Chem Int Ed Engl. 2020. PMID: 32091645
-
Amanita phalloides poisoning: Mechanisms of toxicity and treatment.Food Chem Toxicol. 2015 Dec;86:41-55. doi: 10.1016/j.fct.2015.09.008. Epub 2015 Sep 12. Food Chem Toxicol. 2015. PMID: 26375431 Review.
-
Ribosomal biosynthesis of the cyclic peptide toxins of Amanita mushrooms.Biopolymers. 2010;94(5):659-64. doi: 10.1002/bip.21416. Biopolymers. 2010. PMID: 20564017 Free PMC article. Review.
Cited by
-
Meeting key synthetic challenges in amanitin synthesis with a new cytotoxic analog: 5'-hydroxy-6'-deoxy-amanitin.Chem Sci. 2020 Oct 15;11(43):11927-11935. doi: 10.1039/d0sc04150e. Chem Sci. 2020. PMID: 34094418 Free PMC article.
-
Metabolomic Insights into the Mechanisms of Ganoderic Acid: Protection against α-Amanitin-Induced Liver Injury.Metabolites. 2023 Nov 20;13(11):1164. doi: 10.3390/metabo13111164. Metabolites. 2023. PMID: 37999259 Free PMC article.
-
The occurrence of ansamers in the synthesis of cyclic peptides.Nat Commun. 2022 Oct 30;13(1):6488. doi: 10.1038/s41467-022-34125-8. Nat Commun. 2022. PMID: 36310176 Free PMC article.
-
Synthesis and preliminary evaluation of octreotate conjugates of bioactive synthetic amatoxins for targeting somatostatin receptor (sstr2) expressing cells.RSC Chem Biol. 2021 Oct 7;3(1):69-78. doi: 10.1039/d1cb00036e. eCollection 2022 Jan 5. RSC Chem Biol. 2021. PMID: 35128410 Free PMC article.
-
α-Amanitin aggravates hepatic injury by activating oxidative stress and mitophagy via peroxiredoxin 6 inhibition.Immunol Res. 2025 Mar 19;73(1):64. doi: 10.1007/s12026-025-09619-4. Immunol Res. 2025. PMID: 40108092
References
-
- Wieland T., Faulstich H., Crit. Rev. Biochem. 1978, 5, 185–260. - PubMed
-
- Wieland T., Int. J. Pept. Protein Res. 1983, 22, 257–276. - PubMed
-
- Wienland T., Faulstich H., Experientia 1991, 47, 1186–1193. - PubMed
-
- Cochet-Meilhac M., Chambon P., Biochim. Biophys. Acta Nucleic Acids Protein Synth. 1974, 353, 160–184. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

