Omega-3 and omega-6 polyunsaturated fatty acids for dry eye disease
- PMID: 31847055
- PMCID: PMC6917524
- DOI: 10.1002/14651858.CD011016.pub2
Omega-3 and omega-6 polyunsaturated fatty acids for dry eye disease
Abstract
Background: Polyunsaturated fatty acid (PUFA) supplements, involving omega-3 and/or omega-6 components, have been proposed as a therapy for dry eye. Omega-3 PUFAs exist in both short- (alpha-linolenic acid [ALA]) and long-chain (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) forms, which largely derive from certain plant- and marine-based foods respectively. Omega-6 PUFAs are present in some vegetable oils, meats, and other animal products.
Objectives: To assess the effects of omega-3 and omega-6 polyunsaturated fatty acid (PUFA) supplements on dry eye signs and symptoms.
Search methods: CENTRAL, Medline, Embase, two other databases and three trial registries were searched in February 2018, together with reference checking. A top-up search was conducted in October 2019, but the results have not yet been incorporated.
Selection criteria: We included randomized controlled trials (RCTs) involving dry eye participants, in which omega-3 and/or omega-6 supplements were compared with a placebo/control supplement, artificial tears, or no treatment. We included head-to-head trials comparing different forms or doses of PUFAs.
Data collection and analysis: We followed standard Cochrane methods and assessed the certainty of the evidence using GRADE.
Main results: We included 34 RCTs, involving 4314 adult participants from 13 countries with dry eye of variable severity and etiology. Follow-up ranged from one to 12 months. Nine (26.5%) studies had published protocols and/or were registered. Over half of studies had high risk of bias in one or more domains. Long-chain omega-3 (EPA and DHA) versus placebo or no treatment (10 RCTs) We found low certainty evidence that there may be little to no reduction in dry eye symptoms with long-chain omega-3 versus placebo (four studies, 677 participants; mean difference [MD] -2.47, 95% confidence interval [CI] -5.14 to 0.19 units). We found moderate certainty evidence for a probable benefit of long-chain omega-3 supplements in increasing aqueous tear production relative to placebo (six studies, 1704 participants; MD 0.68, 95% CI 0.26 to 1.09 mm/5 min using the Schirmer test), although we did not judge this difference to be clinically meaningful. We found low certainty evidence for a possible reduction in tear osmolarity (one study, 54 participants; MD -17.71, 95% CI -28.07 to -7.35 mOsmol/L). Heterogeneity was too substantial to pool data on tear break-up time (TBUT) and adverse effects. Combined omega-3 and omega-6 versus placebo (four RCTs) For symptoms (low certainty) and ocular surface staining (moderate certainty), data from the four included trials could not be meta-analyzed, and thus effects on these outcomes were unclear. For the Schirmer test, we found moderate certainty evidence that there was no intergroup difference (four studies, 455 participants; MD: 0.66, 95% CI -0.45 to 1.77 mm/5 min). There was moderate certainty for a probable improvement in TBUT with the PUFA intervention relative to placebo (four studies, 455 participants; MD 0.55, 95% CI 0.04 to 1.07 seconds). Effects on tear osmolarity and adverse events were unclear, with data only available from a single small study for each outcome. Omega-3 plus conventional therapy versus conventional therapy alone (two RCTs) For omega-3 plus conventional therapy versus conventional therapy alone, we found low certainty evidence suggesting an intergroup difference in symptoms favoring the omega-3 group (two studies, 70 participants; MD -7.16, 95% CI -13.97 to -0.34 OSDI units). Data could not be combined for all other outcomes. Long-chain omega-3 (EPA and DHA) versus omega-6 (five RCTs) For long-chain omega-3 versus omega-6 supplementation, we found moderate certainty evidence for a probable improvement in dry eye symptoms (two studies, 130 participants; MD -11.88, 95% CI -18.85 to -4.92 OSDI units). Meta-analysis was not possible for outcomes relating to ocular surface staining, Schirmer test or TBUT. We found low certainty evidence for a potential improvement in tear osmolarity (one study, 105 participants; MD -11.10, 95% CI -12.15 to -10.05 mOsmol/L). There was low level certainty regarding any potential effect on gastrointestinal side effects (two studies, 91 participants; RR 2.34, 95% CI 0.35 to 15.54).
Authors' conclusions: Overall, the findings in this review suggest a possible role for long-chain omega-3 supplementation in managing dry eye disease, although the evidence is uncertain and inconsistent. A core outcome set would work toward improving the consistency of reporting and the capacity to synthesize evidence.
Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
SMN and KL received salary support from Cochrane Eyes and Vision @ US Project, funded by the National Eye Institute, at the National Institutes of Health. LED is the senior author on two studies considered in this review (Chinnery 2017; Deinema 2017); in view of this, a third (independent) person verified data extraction and risk of bias assessment for these studies.
Figures

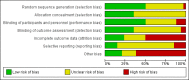









Update of
- doi: 10.1002/14651858.CD011016
References
References to studies included in this review
Aragona 2005 {published data only}
-
- Aragona P, Bucolo C, Spinella R, Giuffrida S, Ferreri G. Systemic omega‐6 essential fatty acid treatment and pge1 tear content in Sjogren's syndrome patients. Investigative Ophthalmology and Visual Science 2005;46(12):4474‐9. - PubMed
Asbell 2018 {published data only}
-
- NCT02128763. Dry Eye Assessment and Management Study (DREAM). https://clinicaltrials.gov/ct2/show/NCT02128763 (first received 1 May 2014).
Barabino 2003 {published data only}
-
- Barabino S, Rolando M, Camicione P, Ravera G, Zanardi S, Giuffrida S, et al. Systemic linoleic and γ‐linolenic acid therapy in dry eye syndrome with an inflammatory component. Cornea 2003;22(2):97‐101. - PubMed
Bhargava 2013 {published data only}
Bhargava 2015a {published data only}
-
- Bhargava R, Kumar P, Phogat H, Kaur A, Kumar M. Oral omega‐3 fatty acids treatment in computer vision syndrome related dry eye. Contact Lens and Anterior Eye 2015;38(3):206‐10. - PubMed
Bhargava 2015b {published data only}
-
- Bhargava R, Kumar P. Oral omega‐3 fatty acid treatment for dry eye in contact lens wearers. Cornea 2015;34(4):413‐20. - PubMed
Bhargava 2016a {published data only}
-
- Bhargava R, Chandra M, Bansal U, Singh D, Ranjan S, Sharma S. A randomized controlled trial of omega 3 fatty acids in Rosacea patients with dry eye symptoms. Current Eye Research 2016;41(10):1274‐80. - PubMed
Bhargava 2016b {published data only}
-
- Bhargava R, Kumar P, Arora Y. Short‐term omega 3 fatty acids treatment for dry eye in young and middle‐aged visual display terminal users. Eye and Contact Lens 2016;42(4):231‐6. - PubMed
Brignole‐Baudouin 2011 {published data only}
-
- Brignole‐Baudouin F, Baudouin C, Aragona P, Rolando M, Labetoulle M, Pisella PJ, et al. A multicentre, double‐masked, randomized, controlled trial assessing the effect of oral supplementation of omega‐3 and omega‐6 fatty acids on a conjunctival inflammatory marker in dry eye patients. Acta Ophthalmologica 2011;89(7):e591‐7. - PubMed
Creuzot 2006 {published data only}
-
- Creuzot C, Passemard M, Viau S, Joffre C, Pouliquen P, Elena PP, et al. Improvement of dry eye symptoms with polyunsaturated fatty acids [Amélioration de la symptomatologie chez des patients atteints de sécheresse oculaire et traités oralement par des acides gras polyinsaturés]. Journal Francais d'Ophthalmologie 2006;29(8):868‐73. - PubMed
Creuzot‐Garcher 2011 {published data only}
-
- Creuzot‐Garcher C, Baudouin C, Labetoulle M, Pisella PJ, Mouriaux F, Meddeb‐Ouertani A, et al. Efficacy assessment of Nutrilarm®, a per os omega‐3 and omega‐6 polyunsaturated essential fatty acid dietary formulation versus placebo in patients with bilateral treated moderate dry eye syndrome [Évaluation de l'efficacité du Nutrilarm®, complément nutritionnel à base d'acides gras essentiels polyinsaturés oméga 3 et oméga 6, versus placebo chez des patients atteints de sécheresse oculaire bilatérale modérée et traitée]. Journal Francais d'Ophthalmologie 2011;34(7):448‐55. - PubMed
Deinema 2017 {published data only}
-
- Chinnery HR, Naranjo Golborne C, Downie LE. Omega‐3 supplementation is neuroprotective to corneal nerves in dry eye disease: a pilot study. Ophthalmic and Physiological Optics 2017;37(4):473‐81. - PubMed
-
- Deinema LA, Vingrys AJ, Wong CY, Jackson DC, Chinnery HR, Downie LE. A randomized, double‐masked, placebo‐controlled clinical trial of two forms of omega‐3 supplements for treating dry eye disease. Ophthalmology 2017;124(1):43‐52. - PubMed
Epitropoulos 2016 {published data only}
Gilbard 2008 {published data only}
-
- Gilbard JP. Prospective, randomized, double‐masked, placebo controlled clinical trial of an omega‐3 supplement for dry eye and dry mouth in patients with Sjögren's disease. American Academy of Ophthalmology; 2008 Nov 8‐11; Atlanta (GA). 2008.
Goyal 2017 {published data only}
-
- Goyal P, Jain AK, Malhotra C. Oral omega‐3 fatty acid supplementation for laser in situ keratomileusis–associated dry eye. Cornea 2017;36(2):169‐75. - PubMed
Kangari 2013 {published data only}
-
- Kangari H, Eftekhari MH, Sardari S, Hashemi H, Salamzadeh J, Ghassemi‐Broumand M, et al. Short‐term consumption of oral omega‐3 and dry eye syndrome. Ophthalmology 2013;120(11):2191‐6. - PubMed
Kawakita 2013 {published data only}
-
- Kawakita T, Kawabata F, Tsuji T, Kawashima M, Shimmura S, Tsubota K. Effects of dietary supplementation with fish oil on dry eye syndrome subjects: randomized controlled trial. Biomedical Research 2013;34(5):215‐20. - PubMed
Kawashima 2016 {published data only}
-
- Kawashima M, Nakamura S, Izuta Y, Inoue S, Tsubota K. Dietary supplementation with a combination of lactoferrin, fish oil,and enterococcus faecium WB2000 for treating dry eye: a rat model and human clinical study. Ocular Surface 2016;14(2):255‐63. - PubMed
Kokke 2008 {published data only}
-
- Kokke KH, Morris JA, Lawrenson JG. Oral omega‐6 essential fatty acid treatment in contact lens associated dry eye. Contact Lens and Anterior Eye 2008;31(3):141‐6. - PubMed
Korb 2015 {published data only}
-
- Korb DR, Blackie CA, Finnemore VM, Douglass T. Effect of using a combination of lid wipes, eye drops, and omega‐3 supplements on meibomian gland functionality in patients with lipid deficient/evaporative dry eye. Cornea 2015;34(4):407‐12. - PubMed
Macsai 2008 {published data only}
Manthorpe 1984 {published data only}
-
- Manthorpe R, Hagen Petersen S, Prause JU. Primary Sjögren's syndrome treated with Efamol/Efavit. A double‐blind cross‐over investigation. Rheumatology International 1984;4(4):165‐7. - PubMed
Mohammadpour 2017 {published data only}
NCT01107964 {published data only}
-
- NCT01107964. Oral omega‐3 fatty acids in the treatment of dry eye syndrome. clinicaltrials.gov/ct2/show/NCT01107964 (first received 21 April 2010).
Oleñik 2013 {published data only}
Oral sea buckthorn oil study 2010 {published data only}
-
- Järvinen RL, Larmo PS, Setälä NL, Yang B, Engblom JR, Viitanen MH, et al. Effects of oral sea buckthorn oil on tear film fatty acids in individuals with dry eye. Cornea 2011;30(9):1013‐9. - PubMed
-
- Larmo PS, Järvinen RL, Setälä NL, Yang B, Viitanen MH, Engblom JR, et al. Oral sea buckthorn oil attenuates tear film osmolarity and symptoms in individuals with dry eye. Journal of Nutrition 2010;140(8):1462‐8. - PubMed
Oxholm 1986 {published data only}
-
- Oxholm P, Manthorpe R, Prause JU, Horrobin D. Patients with primary Sjögren's syndrome treated for two months with evening primrose oil. Scandinavian Journal of Rheumatology 1986;15(2):103‐8. - PubMed
Papas 2007 {published data only}
-
- Papas A, Singh M, Singh M. The effect of a unique omega‐3 supplement on dry mouth and dry eye in Sjögren's patients. Investigative Ophthalmology and Visual Science 2007;48(13):377.
Pinazo‐Durán 2013 {published data only}
-
- Pinazo‐Durán MD, Galbis‐Estrada C, Pons‐Vázquez S, Cantú‐Dibildox J, Marco‐Ramírez C, Benítez‐del‐Castillo J. Effects of a nutraceutical formulation based on the combination of antioxidants and ω‐3 essential fatty acids in the expression of inflammation and immune response mediators in tears from patients with dry eye disorders. Clinical Interventions in Aging 2013;8:139‐48. [DOI: 10.2147/CIA.S40640] - DOI - PMC - PubMed
Pinheiro 2007 {published data only}
-
- Pinheiro MN Jr, dos Santos PM, dos Santos RC, Barros Jde N, Passos LF, Cardoso Neto J. Oral flaxseed oil (Linum usitatissimum) in the treatment for dry‐eye Sjögren's syndrome patients [Uso oral do óleo de linhaça (Linum usitatissimum)no tratamento do olho seco de pacientesportadores da síndrome de Sjögren]. Arquivos Brasileiros de Oftalmologia 2007;70(4):649‐55. - PubMed
Reeder 2006 {published data only}
-
- Reeder RE, Blowe J, Mistry K. Effect of Theratears Nutrition versus flaxseed oil in managing dryness symptoms. www.aaopt.org/detail/knowledge‐base‐article/effect‐theratears‐nutrition‐... (accessed July 31, 2018).
Sheppard 2013 {published data only}
-
- Sheppard JD Jr, Singh R, McClellan AJ, Weikert MP, Scoper SV, Joly TJ, et al. Long‐term supplementation with n‐6 and n‐3 PUFAs improves moderate‐to‐severe keratoconjunctivitis sicca: a randomized double‐blind clinical trial. Cornea 2013;32(10):1297‐304. - PubMed
Theander 2002 {published data only}
-
- Theander E, Horrobin DF, Jacobsson LT, Manthorpe R. Gammalinolenic acid treatment of fatigue associated with primary Sjögren's syndrome. Scandinavian Journal of Rheumatology 2002;31(2):72‐9. - PubMed
Wojtowicz 2011 {published data only}
-
- Wojtowicz JC, Butovich I, Uchiyama E, Aronowicz J, Agee S, McCulley JP. Pilot, prospective, randomized, double‐masked, placebo‐controlled clinical trial of an omega‐3 supplement for dry eye. Cornea 2011;30(3):308‐14. - PubMed
References to studies excluded from this review
Ahluwalia 2001 {published data only}
-
- Ahluwalia P, Anderson DF, Wilson SJ, McGill JI, Church MK. Nedocromil sodium and levocabastine reduce the symptoms of conjunctival allergen challenge by different mechanisms. Journal of Allergy and Clinical Immunology 2001;108(3):449‐54. - PubMed
Arici 2006 {published data only}
-
- Arici MK, Erdogan H, Toker I, Vural A, Topalkara A. The effect of latanoprost, bimatoprost, and travoprost on intraocular pressure after cataract surgery. Journal of Ocular Pharmacology and Therapeutics 2006;22(1):34‐40. - PubMed
Costa 2012 {published data only}
Downie 2018b {published data only}
-
- Downie LE, Gad A, Wong CY, Gray JHV, Zeng W, Jackson DC, et al. Modulating contact lens discomfort with anti‐Inflammatory approaches: A randomized controlled trial. Investigative Ophthalmology & Visual Science 2018;59(8):3755‐66. - PubMed
Gadaria‐Rathod 2013 {published data only}
Hom 2016 {published data only}
-
- Hom MM. Baseline characteristics in the dry eye assessment and management (DREAM) study. Investigative Ophthalmology and Visual Science 2016;57(12):2844.
Jackson 2011 {published data only}
Kawabata 2011 {published data only}
-
- Kawabata F, Tsuji T. Effects of dietary supplementation with a combination of fish oil, bilberry extract, and lutein on subjective symptoms of asthenopia in humans. Biomedical Research 2011;32(6):387‐93. - PubMed
Kaya 2016 {published data only}
Kwon 2017 {published data only}
Macrì 2001 {published data only}
-
- Macrì A, et al. Effect of linoleic acid and gamma‐linolenic acid on tear production, clearance and the ocular surface after PRK. Investigative Ophthalmology and Visual Science 2001; Vol. 42:ARVO Abstract 2658.
Macrì 2002 {published data only}
-
- Macrì A, Amico V, Cro M, Giuffrida S. Effects and compliance of a new oral formulation containing linoleic acid and gamma‐linolenic acid on tear production, tear fluorescein clearance and ocular surface after PRK. Investigative Ophthalmology and Visual Science 2002; Vol. 43:ARVO E‐Abstract 2105.
Macrì 2003 {published data only}
-
- Macrì A, Giuffrida S, Amico V, Lester M, Traverso CE. Effect of linoleic acid and gamma‐linolenic acid on tear production, tear clearance and on the ocular surface after photorefractive keratectomy. Graefe's Archive for Clinical and Experimental Ophthalmology 2003;241(7):561‐6. - PubMed
McMonnies 2019 {published data only}
-
- McMonnies CW. Can dietary approaches to the treatment of dry eye disease be improved?. The Ocular Surface 2019; Vol. 17, issue 3:370‐1. - PubMed
NCT00803452 {published data only}
-
- NCT00803452. Lipids of the human tear film and their effect on tear stability. clinicaltrials.gov/ct2/show/NCT00803452 (first received December 5, 2008).
NCT01059019 {published data only}
-
- NCT01059019. Epithelial healing and visual outcomes using omega‐3 therapy before and after Photorefractive Keratectomy (PRK) surgery (Omega‐3). https://clinicaltrials.gov/ct2/show/NCT01059019 (first received January 29, 2010).
NCT01213342 {published data only}
-
- NCT01213342. Omega‐3 fatty acid supplements and dry eye. clinicaltrials.gov/ct2/show/NCT01213342 (first received October 4, 2010).
NCT01364311 {published data only}
-
- NCT01364311. Treatment of dry eye with supplements. https://clinicaltrials.gov/ct2/show/NCT01364311 (first received June 2, 2011).
NCT01630551 {published data only}
-
- NCT01630551. Omega 3 supplementation and ocular surface disease in glaucoma. clinicaltrials.gov/ct2/show/NCT01630551 (first received June 28, 2012).
No author listed {published data only}
-
- Dry eye: fish oil capsules are not better than placebo. Pharmazeutische Zeitung 2018;163(16).
No authors listed {published data only}
-
- Omega‐3 fatty acid supplements do not improve symptoms of dry eye disease. Drug and Therapeutics Bulletin 2018;56(12):144. - PubMed
Ong 2013 {published data only}
-
- Ong NH, Purcell TL, Roch‐Levecq AC, Wang D, Isidro MA, Bottos KM, et al. Epithelial healing and visual outcomes of patients using omega‐3 oral nutritional supplements before and after photorefractive keratectomy: a pilot study. Cornea 2013;32(6):761‐5. - PubMed
Pinna 2007 {published data only}
-
- Pinna A, Piccinini P, Carta F. Effect of oral linoleic and gamma‐linolenic acid on meibomian gland dysfunction. Cornea 2007;26(3):260‐4. - PubMed
Querques 2007 {published data only}
-
- Querques G, Russo V, Barone A, Iaculli C, Delle Noci N. Efficacy of omega‐6 essential fatty acid treatment before and after photorefractive keratectomy [Efficacité du traitement avec des acides gras essentiels oméga‐6 administrés avant et aprés la kératectomie photoréfractive]. Journal Français d'Ophtalmologie 2008;31(3):282‐6. - PubMed
Scuderi 2012 {published data only}
-
- Scuderi G, Contestabile MT, Gagliano C, Iacovello D, Scuderi L, Avitabile T. Effects of phytoestrogen supplementation in postmenopausal women with dry eye syndrome: a randomized clinical trial. Canadian Journal of Ophthalmology 2012;47(6):489‐92. - PubMed
Sharma 2006 {published data only}
-
- Sharma AK, Raj H, Pandita A, Tandon VR, Mahajan A. Gamma linolenic acid in dry eye. JK Science 2008;8(1):24‐7.
Tellez‐Vazquez 2016 {published data only}
Wang 2016 {published data only}
-
- Wang L, Chen X, Hao J, Yang L. Proper balance of omega‐3 and omega‐6 fatty acid supplements with topical cyclosporine attenuated contact lens‐related dry eye syndrome. Inflammopharmacology 2016;24(6):389‐96. - PubMed
Yoon 2012 {published data only}
-
- Yoon S, Gu N, Kim S, Soo Lim K. Ocular tolerability and safety assessment of DA‐6034 ophthalmic solution in healthy subjects. Clinical Pharmacology and Therapeutics 2012;91:S56.
References to studies awaiting assessment
Follow‐up reports from the DREAM study {published data only}
-
- Hussain M, Shtein RM, Pistilli M, Maguire MG, Oydanich M, Asbell PA, Dream Study Research Group. The Dry Eye Assessment and Management (DREAM) extension study ‐ A randomized clinical trial of withdrawal of supplementation with omega‐3 fatty acid in patients with dry eye disease. In: Ocular Surface; 2019;16:Pismo Beach (CA). - PMC - PubMed
-
- Yu Y, Schmucker E, Maguire MG, Sutphin JE, Asbell PA. Correlation of clinical and keratographic assessments of tear stability and tear production at baseline in the DRy Eye Assessment and Management (DREAM©) Study. Investigative Ophthalmology and Visual Science 2018;59(9).
Hom 2017 {published data only}
-
- Hom M, Berdy G, Downie L, El‐Harazi S, Verachtert A, Liu H, Carlisle‐Wilcox C, Simmons P, Vehige J. Clinical evaluation of a novel lipid‐containing lubricant eye drop with omega‐3 oil and trehalose. Investigative Ophthalmology & Visual Science 2017;58(8).
Laihia 2019 {published data only}
NCT02980224 {published data only}
-
- NCT02980224. Study of the safety and efficacy of OmegaD softgels in the treatment of dry eye disease. https://clinicaltrials.gov/ct2/show/NCT02980224 (first received December 2, 2016).
NCT03569202 {published data only}
-
- NCT03569202. Piiloset trehalose emulsion eye drop study in moderate or severe dry eye. https://clinicaltrials.gov/ct2/show/NCT03569202 (first received June 26, 2018).
References to ongoing studies
ACTRN12610000991011 {published data only}
-
- ACTRN12610000991011. Dietary supplements and ocular (eye) comfort [A randomised placebo‐controlled double‐masked study to investigate the effects of dietary supplementation with a combination of omega oils on ocular comfort including symptoms of dry eye]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12610000991011 (first received November 16, 2010).
IRCT201012265467N {published data only}
-
- IRCT201012265467N. The impact of omega‐3 on dry eye. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201012265467N1 (first received April 3, 2012).
IRCT2013062413567N4 {published data only}
-
- IRCT2013062413567N4. Impact of omega‐3 supplement on dry eye treatment [Clinical trial comparing the effects of omega‐3 supplements and artificial tear drops on dry eye symptoms after surgery, cataract emulsification methods]. en.irct.ir/trial/13389 (first received January 11, 2013).
IRCT20180806040722N1 {published data only}
-
- IRCT20180806040722N1. Effect of complementary supplementation Camelina Oil on the improvement of dry eye disease in patients with dry eye. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20180806040722N1 (first received April 22, 2019).
IRCT20181028041487N1 {published data only}
-
- IRCT20181028041487N1. Evaluation the effect of intra nasal viola odorata almond oil drop on dry eye disease. https://en.irct.ir/trial/35071 (first received January 28, 2019).
ISRCTN10758297 {published data only}
-
- ISRCTN10758297. The effect of a unique omega‐3 supplement on dry mouth and dry eye in Sjogren's patients. http://www.isrctn.com/ISRCTN10758297 (first received February 7, 2007).
ISRCTN11797545 {published data only}
-
- ISRCTN11797545. Evaluation of combined Eyepeace and Eye Nutrients study. http://www.isrctn.com/ISRCTN11797545 (first received February 9, 2018).
ISRCTN17233445 {published data only}
-
- ISRCTN17233445. Oral supplementation of omega‐3 and omega‐6 in dry eye syndrome. isrctn.com/ISRCTN17233445 (first received November 28, 2008).
NCT00344721 {published data only}
-
- NCT00344721. A placebo controlled double masked clinical assessment study of essential fatty acid supplement and Its effect on patients with apparent aqueous deficient dry eye syndrome. clinicaltrials.gov/ct2/show/NCT00344721 (first received June 27, 2006).
NCT00357201 {published data only}
-
- NCT00357201. Efficacy of T1675 versus placebo in patients with bilateral treated moderate dry eye syndrome. clinicaltrials.gov/ct2/show/NCT00357201 (first received July 27, 2006).
NCT01102257 {published data only}
-
- NCT01102257. Dry eye assessment and management: feasibility study (DREAM). clinicaltrials.gov/ct2/show/NCT01102257 (first received August 23, 2012).
NCT01733745 {published data only}
-
- NCT01733745. SYSTANE family ‐ meibomian deficiency (M‐12‐077). clinicaltrials.gov/ct2/show/NCT01733745 (first received July 3, 2014).
NCT01880463 {published data only}
-
- NCT01880463. Dry eye disease in the vitamin D and omega‐3 Trial (VITAL). clinicaltrials.gov/ct2/show/NCT01880463 (first received June 19, 2013).
NCT02014922 {published data only}
-
- NCT02014922. A study to determine the relief of dry eye symptoms with the use of TheraTears® products (DUNLIN). https://clinicaltrials.gov/ct2/show/NCT02014922 (first received December 18, 2013).
NCT02802150 {published data only}
-
- NCT02802150. The effect of oral Zanthoxylum Schinifolium seed oil in individuals with dry eye disease. https://clinicaltrials.gov/ct2/show/NCT02802150 (first received June 16, 2016).
NCT02871440 {published data only}
-
- NCT02871440. A two comparator, controlled phase 3 study in patients with and without evaporative dry eye. clinicaltrials.gov/ct2/show/NCT02871440 (first received August 18, 2016).
NCT02908282 {published data only}
-
- NCT02908282. Topical omega‐3 fatty acids (REMOGEN OMEGA) in the treatment of dry eye (REMOTOP). clinicaltrials.gov/ct2/show/NCT02908282 (first received September 20, 2016).
NCT03141931 {published data only}
-
- NCT03141931. The effects of dietary supplementation with a combination of flaxseed oil, borage oil and fish oil omega‐3 fatty acids on ocular comfort including symptoms of dry eye. clinicaltrials.gov/ct2/show/NCT03141931 (first received May 5, 2017).
NCT03265327 {published data only}
-
- NCT03265327. Effect of an oral supplement containing omega‐3 and omega‐6 on dry eye symptoms (TURMERIC). clinicaltrials.gov/ct2/show/NCT03265327 (first received August 29, 2017).
NCT03460548 {published data only}
-
- NCT03460548. REMOGEN® omega versus Cationorm® in the treatment of patients suffering from dry eye. https://clinicaltrials.gov/ct2/show/NCT03460548 (first received March 9, 2018).
Additional references
AAO 2013
-
- American Academy of Ophthalmology (AAO) Cornea/ External Disease Panel. Preferred practice pattern for dry eye syndrome. www.aao.org/preferred‐practice‐pattern/dry‐eye‐syndrome‐ppp‐2013 2013; Vol. (accessed July 14, 2018).
Bhandari 2004
Bistrian 2018
-
- Bistrian BR. Letter to the Editor re: n‐3 fatty acid supplementation and dry eye disease. New England Journal of Medicine 2018;379:690‐1. - PubMed
Brasky 2013
Brenna 2002
-
- Brenna JT. Efficiency of conversion of alpha‐linolenic acid to long chain n‐3 fatty acids in man. Current Opinion in Clinical Nutrition and Metabolic Care 2002;5(2):127‐32. - PubMed
Bron 2003
-
- Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 2003;22(7):640‐50. - PubMed
Brouwer 2004
-
- Brouwer IA, Katan MB, Zock PL. Dietary alpha‐linolenic acid is associated with reduced risk of fatal coronary heart disease, but increased prostate cancer risk: a meta‐analysis. Journal of Nutrition 2004;134(4):919‐22. - PubMed
Buckley 2004
-
- Buckley MS, Goff AD, Knapp WE. Fish oil interaction with warfarin. Annals of Pharmacotherapy 2004;38(1):50‐2. - PubMed
Caughey 1996
-
- Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ. The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n‐3 fatty acids from vegetable oil or fish oil. American Journal of Clinical Nutrition 1996;63(1):116‐22. - PubMed
Chinnery 2017
-
- Chinnery HR, Naranjo Golborne C, Downie LE. Omega‐3 supplementation is neuroprotective to corneal nerves in dry eye disease: a pilot study. Ophthalmic and Physiological Optics 2017;37(4):473‐81. - PubMed
Craig 2017
-
- Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, et al. TFOS DEWS II Definition and Classification Report. Ocular Surface 2017;15(3):276‐83. - PubMed
Dalgado 2017
Deeks 2017
-
- Deeks JJ, Higgins JPT, Altman DG (editors), on behalf of the Cochrane Statistical Methods Group. Chapter 9. Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook..
Downie 2013
-
- Downie LE, Keller PR, Vingrys AJ. An evidence‐based analysis of Australian optometrists' dry eye practices. Optometry and Vision Science 2013;90(12):1385‐95. - PubMed
Downie 2015a
-
- Downie LE, Keller PR. A pragmatic approach to dry eye diagnosis: evidence into practice. Optometry and Vision Science 2015;92(12):1189‐97. - PubMed
Downie 2015b
-
- Downie LE, Keller PR. A pragmatic approach to the management of dry eye disease: evidence into practice. Optometry and Vision Science 2015;92(9):957‐66. - PubMed
Downie 2015c
-
- Downie LE. Automated tear film surface quality breakup time as a novel clinical marker for tear hyperosmolarity in dry eye disease. Investigative Ophthalmology and Visual Science 2015;56(12):7260‐8. - PubMed
Downie 2018a
-
- Downie LE, Gad A, Wong CY, Gray JH, Zeng W, Jackson DC, et al. Modulating contact lens discomfort with anti‐inflammatory approaches: a randomized controlled trial. Investigative Ophthalmology and Visual Science 2018;59(8):3755‐66. - PubMed
Dyerberg 2010
-
- Dyerberg J, Madsen P, Moller JM, Aardestrup I, Schmidt EB. Bioavailability of marine n‐3 fatty acid formulations. Prostaglandins, Leukotrienes and Essential Fatty Acids 2010;83(3):137‐41. - PubMed
Graf 2010
-
- Graf BA, Duchateau GS, Patterson AB, Mitchell ES, Bruggen P, Koek JH, et al. Age dependent incorporation of 14 C‐DHA into rat brain and body tissues after dosing various 14C‐DHA‐esters. Prostaglandins Leukotrienes and Essential Fatty Acids 2010;83(2):89‐96. - PubMed
Hampel 2015
Higgins 2017
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8. Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Hoffmann 2014
-
- Hoffmann TC, Glasziou PP, Milne R, Moher D, Barbour V, Johnston M, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. - PubMed
IOM 2002
-
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty acids, Cholesterol, Protein and Amino Acids. Washington, DC: National Academies Press, 2002. - PubMed
Jackson 2016
-
- Jackson DC, Zeng W, Wong C, Vingrys AJ, Downie LE. Tear interferon‐gamma as a biomarker for evaporative dry eye disease. Investigative Ophthalmology and Visual Science 2016;57:4824‐30. - PubMed
James 2000
-
- James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. American Journal of Clinical Nutrition 2000;71(1 Suppl):S343‐8. - PubMed
Johnson 2009
-
- Johnson ME. The association between symptoms of discomfort and signs in dry eye. Ocular Surface 2009;7(4):199‐211. - PubMed
Jones 2017
-
- Jones L, Downie LE, Korb D, Benitez‐Del‐Castillo JM, Dana R, Deng SX, et al. TFOS DEWS II Management and Therapy Report. Ocular Surface 2017;15:575‐628. - PubMed
Karakus 2018
-
- Karakus S, Agrawal D, Hindman HB, Henrich C, Ramulu PY, Akpek E. Effects of prolonged reading on dry eye. Ophthalmology 2018;125(10):1500‐5. - PubMed
Kris‐Etherton 2007
-
- Kris‐Etherton PM, Innis S, American Dietetic Association, Dietitians of Canada. Position of the American Dietetic Association and Dietitians of Canada: dietary fatty acids. Journal of the American Dietetic Association 2007;107(9):1599‐611. - PubMed
Lemp 1995
-
- Lemp MA. Report of the National Eye Institute/Industry workshop on clinical trials in dry eyes. CLAO Journal 1995;21(4):221‐32. - PubMed
Lemp 2012
-
- Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous‐deficient and evaporative dry eye in a clinic‐based patient cohort: a retrospective study. Cornea 2012;31(5):472‐8. - PubMed
Li 2010
Li 2011
-
- Li M, Gong L, Sun X, Chapin WJ. Anxiety and depression in patients with dry eye syndrome. Current Eye Research 2011;36(1):1‐7. - PubMed
Li 2014
-
- Li Z, Choi JH, Oh HJ, Park SH, Lee JB, Yoon KC. Effects of eye drops containing a mixture of omega‐3 essential fatty acids and hyaluronic acid on the ocular surface in desiccating stress‐induced murine dry eye. Current Eye Research 2014;39(9):871‐8. - PubMed
Liu 2014
Liu 2016
Maguire 2018
-
- Maguire MG, Asbell PA. Author's response to: Bistrian BR re: n‐3 fatty acid supplementation and dry eye disease. New England Journal of Medicine 2018;379:690‐1. - PubMed
Massingale 2008
-
- Massingale ML, Li X, Vallabhajosyula M, Chen D, Wei Y, Asbell PA. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea 2008;28(9):1023‐7. - PubMed
Meyer 2016
Miljanovic 2005
Miller 2010
-
- Miller KL, Walt JG, Mink DR, Satram‐Hoang S, Wilson SE, Perry HD, et al. Minimal clinically important difference for the ocular surface disease index. JAMA Ophthalmology 2010;128(1):94‐101. - PubMed
Molina‐Levya 2017
-
- Molina‐Leyva I, Molina‐Leyva A, Bueno‐Cavanillas A. Efficacy of nutritional supplementation with omega‐3 and omega‐6 fatty acids in dry eye syndrome: a systematic review of randomized clinical trials. Acta Ophthalmologica 2017;95(8):e677‐e85. - PubMed
Nichols 2004
-
- Nichols KK, Nichols JJ, Mitchell GL. The lack of association between signs and symptoms in patients with dry eye disease. Cornea 2004;23(8):762‐70. - PubMed
Novack 2017
Pflugfelder 2008
Prinsen 2014
-
- Prinsen CA, Vohra S, Rose MR, King‐Jones S, Ishaque S, Bhaloo Z, Adams D, Terwee CB. Core Outcome Measures in Effectiveness Trials (COMET) initiative: protocol for an international Delphi study to achieve consensus on how to select outcome measurement instruments for outcomes included in a ‘core outcome set’. Trials 2014;15:247. [DOI: 10.1186/1745-6215] - DOI - PMC - PubMed
Reveiz 2010
-
- Reveiz L, Cortes‐Jofre M, Lobos CA, Nicita G, Ciapponi A, Garcia‐Dieguez M, et al. Influence of trial registration on reporting quality of randomized trials: study from highest ranked journals. Journal of Clinical Epidemiology 2010;63(11):1216‐22. - PubMed
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosenberg 2010
-
- Rosenberg ES, Asbell PA. Essential fatty acids in the treatment of dry eye. Ocular Surface 2010;8(1):18‐28. - PubMed
Saldanha 2018
Schaumberg 2003
-
- Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. American Journal of Ophthalmology 2003;136(2):318‐26. - PubMed
Schaumberg 2009
Schiffman 2003
-
- Schiffman RM, Walt JG, Jacobsen G, Doyle JJ, Lebovics G, Sumner W. Utility assessment among patients with dry eye disease. Ophthalmology 2003;110(7):1412‐9. - PubMed
Schünemann 2017
-
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Akl E, Guyatt GH, on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11. Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Simopoulos 2002
-
- Simopoulos AP. The importance of the ratio of omega‐6/omega‐3 essential fatty acids. Biomedicine and Pharmacotherapy 2002;56(8):365‐79. - PubMed
Solomon 2001
-
- Solomon A, Dursun D, Liu Z, Xie Y, Macri A, Pflugfelder SC. Pro‐ and anti‐inflammatory forms of interleukin‐1 in the tear fluid and conjunctiva of patients with dry‐eye disease. Investigative Ophthalmology and Visual Science 2001;42(10):2283‐92. - PubMed
Stapleton 2017
-
- Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, et al. TFOS DEWS II Epidemiology Report. Ocular Surface 2017;15(3):334‐65. - PubMed
Stern 1998
-
- Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea 1998;17(6):548‐9. - PubMed
Stevenson 2013
Sullivan 2002
-
- Sullivan BD, Cermak JM, Sullivan RM, Papas AS, Evans JE, Dana RM, Sullivan DA. Correlations between nutrient intake and the polar lipid profiles of meibomian gland secretions in women with Sjögren’s syndrome. In: Sullivan DA, Stern ME, Tsubota K, Dartt DA, Sullivan RM, Bromberg BB editor(s). Lacrimal Gland, Tear Film, and Dry Eye Syndromes 3. Advances in Experimental Medicine and Biology. Vol. 506, Boston, MA: Springer, 2002. - PubMed
Surette 2008
Szymanski 2010
-
- Szymanski KM, Wheeler DC, Mucci LA. Fish consumption and prostate cancer risk: a review and meta‐analysis. American Journal of Nutrition 2010;92(5):1223‐33. - PubMed
Terry 1993
-
- Terry RL, Schnider CM, Holden BA, Cornish R, Grant T, Sweeney D, et al. CCLRU standards for success of daily and extended wear contact lenses. Optometry and Vision Science 1993;70(3):234‐43. - PubMed
Uchino 2012
-
- Uchino Y, Uchino M, Dogru M, Ward S, Yokoi N, Tsubota K. Changes in dry eye diagnostic status following implementation of revised Japanese dry eye diagnostic criteria. Japanese Journal of Ophthalmology 2012;56(1):8‐13. - PubMed
van Bijsterveld 1969
-
- Bijsterveld OP. Diagnostic tests in the sicca syndrome. Archives of Ophthalmology 1969;82(1):10‐4. - PubMed
Walter 2016
Wang 2018
-
- Wang TM, Craig JP. Core outcome sets for clinical trials in dry eye disease. JAMA Ophthalmology 2018;136(10):1180‐1. - PubMed
Wolffsohn 2017
-
- Wolffsohn JS, Arita R, Chalmers R, Djalilian A, Dogru M, Dumbleton K, et al. TFOS DEWS II Diagnostic Methodology report. Ocular Surface 2017;15(3):539‐74. - PubMed
Yu 2011
-
- Yu J, Asche CV, Fairchild CJ. The economic burden of dry eye disease in the United States: a decision tree analysis. Cornea 2011;30(4):379‐87. - PubMed
Zhang 2019a
Zhang 2019b
-
- Zhang AC, Silva MEH, MacIsaac RJ, Roberts L, Kamel J, Craig JP, Busija L, Downie LE. Omega‐3 polyunsaturated fatty acid oral supplements for improving peripheral nerve health: a systematic review and meta‐analysis. Nutrition Reviews 2019:pii: nuz054. - PubMed
Zhu 2014
-
- Zhu W, Wu Y, Li G, Wang J, Li X. Efficacy of polyunsaturated fatty acids for dry eye syndrome: a meta‐analysis of randomized controlled trials. Nutrion Reviews 2014;72(10):662‐71. - PubMed
Ziemanski 2018
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

