The Striking Flower-in-Flower Phenotype of Arabidopsis thaliana Nossen (No-0) is Caused by a Novel LEAFY Allele
- PMID: 31847079
- PMCID: PMC6963406
- DOI: 10.3390/plants8120599
The Striking Flower-in-Flower Phenotype of Arabidopsis thaliana Nossen (No-0) is Caused by a Novel LEAFY Allele
Abstract
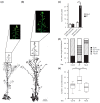
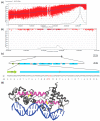
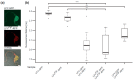
The transition to reproduction is a crucial step in the life cycle of any organism. In Arabidopsis thaliana the establishment of reproductive growth can be divided into two phases: Firstly, cauline leaves with axillary meristems are formed and internode elongation begins. Secondly, lateral meristems develop into flowers with defined organs. Floral shoots are usually determinate and suppress the development of lateral shoots. Here, we describe a transposon insertion mutant in the Nossen accession with defects in floral development and growth. Most strikingly is the outgrowth of stems from the axillary bracts of the primary flower carrying secondary flowers. Therefore, we named this mutant flower-in-flower (fif). However, the transposon insertion in the annotated gene is not the cause for the fif phenotype. By means of classical and genome sequencing-based mapping, the mutation responsible for the fif phenotype was found to be in the LEAFY gene. The mutation, a G-to-A exchange in the second exon of LEAFY, creates a novel lfy allele and results in a cysteine-to-tyrosine exchange in the α1-helix of LEAFY's DNA-binding domain. This exchange abolishes target DNA-binding, whereas subcellular localization and homomerization are not affected. To explain the strong fif phenotype against these molecular findings, several hypotheses are discussed.
Keywords: Arabidopsis thaliana; DNA-binding; Ds transposon; LEAFY; classical/sequencing-based mapping; floral development; flower morphology.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




Similar articles
-
Non-inductive conditions expose the cryptic bract of flower phytomeres in Arabidopsis thaliana.Plant Signal Behav. 2015;10(4):e1010868. doi: 10.1080/15592324.2015.1010868. Plant Signal Behav. 2015. PMID: 25924005 Free PMC article.
-
The role of two LEAFY paralogs from Idahoa scapigera (Brassicaceae) in the evolution of a derived plant architecture.Plant J. 2007 Jul;51(2):211-9. doi: 10.1111/j.1365-313X.2007.03148.x. Epub 2007 Jun 8. Plant J. 2007. PMID: 17559504
-
LEAFY and Polar Auxin Transport Coordinately Regulate Arabidopsis Flower Development.Plants (Basel). 2014 Apr 30;3(2):251-65. doi: 10.3390/plants3020251. Plants (Basel). 2014. PMID: 27135503 Free PMC article.
-
The poetry of reproduction: the role of LEAFY in Arabidopsis thaliana flower formation.Int J Dev Biol. 2012;56(4):207-21. doi: 10.1387/ijdb.113450ns. Int J Dev Biol. 2012. PMID: 22451042 Review.
-
Turning Meristems into Fortresses.Trends Plant Sci. 2019 May;24(5):431-442. doi: 10.1016/j.tplants.2019.02.004. Epub 2019 Mar 7. Trends Plant Sci. 2019. PMID: 30853243 Review.
Cited by
-
Roles and evolution of four LEAFY homologs in floral patterning and leaf development in woodland strawberry.Plant Physiol. 2023 May 2;192(1):240-255. doi: 10.1093/plphys/kiad067. Plant Physiol. 2023. PMID: 36732676 Free PMC article.
-
Three-Fluorophore FRET Enables the Analysis of Ternary Protein Association in Living Plant Cells.Plants (Basel). 2022 Oct 6;11(19):2630. doi: 10.3390/plants11192630. Plants (Basel). 2022. PMID: 36235497 Free PMC article.
-
RNAi Suppression of LEAFY Gives Stable Floral Sterility, and Reduced Growth Rate and Leaf Size, in Field-Grown Poplars.Plants (Basel). 2021 Aug 3;10(8):1594. doi: 10.3390/plants10081594. Plants (Basel). 2021. PMID: 34451639 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

