MicroRNAs as Therapeutic Targets and Clinical Biomarkers in Atherosclerosis
- PMID: 31847094
- PMCID: PMC6947565
- DOI: 10.3390/jcm8122199
MicroRNAs as Therapeutic Targets and Clinical Biomarkers in Atherosclerosis
Abstract
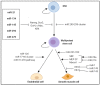
Atherosclerotic cardiovascular disease remains the leading cause of morbidity and mortality worldwide. Atherosclerosis develops over several decades and is mediated by a complex interplay of cellular mechanisms that drive a chronic inflammatory milieu and cell-to-cell interactions between endothelial cells, smooth muscle cells and macrophages that promote plaque development and progression. While there has been significant therapeutic advancement, there remains a gap where novel therapeutic approaches can complement current therapies to provide a holistic approach for treating atherosclerosis to orchestrate the regulation of complex signalling networks across multiple cell types and different stages of disease progression. MicroRNAs (miRNAs) are emerging as important post-transcriptional regulators of a suite of molecular signalling pathways and pathophysiological cellular effects. Furthermore, circulating miRNAs have emerged as a new class of disease biomarkers to better inform clinical diagnosis and provide new avenues for personalised therapies. This review focusses on recent insights into the potential role of miRNAs both as therapeutic targets in the regulation of the most influential processes that govern atherosclerosis and as clinical biomarkers that may be reflective of disease severity, highlighting the potential theranostic (therapeutic and diagnostic) properties of miRNAs in the management of cardiovascular disease.
Keywords: angiogenesis; endothelial dysfunction; foam cell formation; inflammation; oxidative stress; plaque rupture; plaque stability; smooth muscle cells; vasa vasorum.
Conflict of interest statement
The authors declare no conflict of interest associated with this article.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

