Strategies for Molecular Imprinting and the Evolution of MIP Nanoparticles as Plastic Antibodies-Synthesis and Applications
- PMID: 31847152
- PMCID: PMC6940816
- DOI: 10.3390/ijms20246304
Strategies for Molecular Imprinting and the Evolution of MIP Nanoparticles as Plastic Antibodies-Synthesis and Applications
Abstract
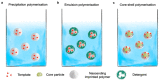
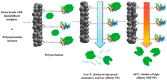
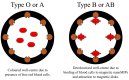
Materials that can mimic the molecular recognition-based functions found in biology are a significant goal for science and technology. Molecular imprinting is a technology that addresses this challenge by providing polymeric materials with antibody-like recognition characteristics. Recently, significant progress has been achieved in solving many of the practical problems traditionally associated with molecularly imprinted polymers (MIPs), such as difficulties with imprinting of proteins, poor compatibility with aqueous environments, template leakage, and the presence of heterogeneous populations of binding sites in the polymers that contribute to high levels of non-specific binding. This success is closely related to the technology-driven shift in MIP research from traditional bulk polymer formats into the nanomaterial domain. The aim of this article is to throw light on recent developments in this field and to present a critical discussion of the current state of molecular imprinting and its potential in real world applications.
Keywords: assay; molecular imprinting; nanoMIP; protein imprinting; sensor; therapeutic agent.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
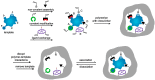
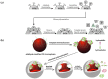

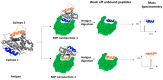
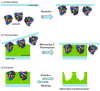
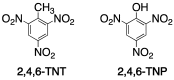


References
-
- MIPdatabase. [(accessed on 10 December 2019)]; Available online: https://www.mipdatabase.com.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

