Genetically Engineered Lung Cancer Cells for Analyzing Epithelial-Mesenchymal Transition
- PMID: 31847480
- PMCID: PMC6953058
- DOI: 10.3390/cells8121644
Genetically Engineered Lung Cancer Cells for Analyzing Epithelial-Mesenchymal Transition
Abstract
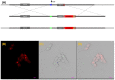
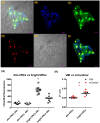
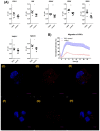
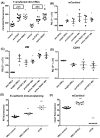
Cell plasticity, defined as the ability to undergo phenotypical transformation in a reversible manner, is a physiological process that also exerts important roles in disease progression. Two forms of cellular plasticity are epithelial-mesenchymal transition (EMT) and its inverse process, mesenchymal-epithelial transition (MET). These processes have been correlated to the poor outcome of different types of neoplasias as well as drug resistance development. Since EMT/MET are transitional processes, we generated and validated a reporter cell line. Specifically, a far-red fluorescent protein was knocked-in in-frame with the mesenchymal gene marker VIMENTIN (VIM) in H2170 lung cancer cells. The vimentin reporter cells (VRCs) are a reliable model for studying EMT and MET showing cellular plasticity upon a series of stimulations. These cells are a robust platform to dissect the molecular mechanisms of these processes, and for drug discovery in vitro and in vivo in the future.
Keywords: cancer cell line; epithelial–mesenchymal transition (EMT); mesenchymal–epithelial transition (MET); reporter; vimentin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Mittal V. Advances in Experimental Medicine and Biology. Volume 890. Springer; Cham, Switzerland: 2016. Epithelial Mesenchymal Transition in Aggressive Lung Cancers; pp. 37–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

