Eribulin plus trastuzumab in pretreated HER2-positive advanced breast cancer patients: safety and efficacy. An Italian experience
- PMID: 31847742
- PMCID: PMC7372580
- DOI: 10.1177/0300891619887225
Eribulin plus trastuzumab in pretreated HER2-positive advanced breast cancer patients: safety and efficacy. An Italian experience
Abstract
Background: Chemotherapy plus targeted therapy is the established treatment for human epidermal growth factor receptor 2 (HER2)-overexpressing breast cancer (BC). Limited data regarding the safety and activity of the combination of eribulin and trastuzumab (E/T) in pretreated HER2-positive advanced BC (ABC) are available. The aim of this observational, retrospective, multicenter study was to examine the tolerability and the clinical activity of E/T in this setting.
Methods: Patients treated with eribulin mesylate plus standard dose of trastuzumab were included. Data on overall response rate (ORR), progression-free survival (PFS), overall survival (OS), and safety were reported.
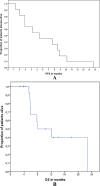
Results: Between October 2012 and November 2015, 24 consecutive patients with HER2-positive ABC were included. All patients were heavily pretreated: the median number of prior chemotherapy regimens for ABC was 3 (range 2-9). The median number of cycles with E/T was 11.5 (range 2-26). The ORR was 41.7%. Median PFS was 5.4 months, median postprogression survival was 5.4 months, and median OS was 8 months. Neutropenia was the most common grade 3/4 clinical adverse event (16.7%).
Conclusions: Tolerability and clinical activity of the E/T combination schedule are encouraging. The results of this study indicate that this combination might be considered for treatment of pretreated HER2 ABC.
Keywords: Eribulin; HER2-positive breast cancer; trastuzumab.
Conflict of interest statement
Figures
References
-
- Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol 2016; 3: 524–548. - PMC - PubMed
-
- Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/Neu oncogene. Science 1987; 235: 177–182. - PubMed
-
- Andrulis IL, Bull SB, Blackstein ME, et al. Neu/erbB-2 amplification identifies a poor-prognosis group of women with node-negative breast cancer: Toronto Breast Cancer Study Group. J Clin Oncol 1998; 16: 1340–1349. - PubMed
-
- Sjogren S, Inganas M, Lindgren A, et al. Prognostic and predictive value of cerbB-2 overexpression in primary breast cancer, alone and in combination with other prognostic markers. J Clin Oncol 1998; 16: 462–469. - PubMed
-
- Andersson M, Lidbrink E, Bjerre K, et al. Phase III randomized study comparing docetaxel plus trastuzumab with vinorelbine plus trastuzumab as first-line therapy of metastatic or locally advanced human epidermal growth factor receptor 2-positive breast cancer: the HERNATA study. J Clin Oncol 2011; 29: 264–271. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous