Carbonic anhydrase 2 (CAII) supports tumor blood endothelial cell survival under lactic acidosis in the tumor microenvironment
- PMID: 31847904
- PMCID: PMC6918655
- DOI: 10.1186/s12964-019-0478-4
Carbonic anhydrase 2 (CAII) supports tumor blood endothelial cell survival under lactic acidosis in the tumor microenvironment
Abstract
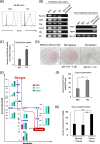
Background: Tumor endothelial cells (TECs) perform tumor angiogenesis, which is essential for tumor growth and metastasis. Tumor cells produce large amounts of lactic acid from glycolysis; however, the mechanism underlying the survival of TECs to enable tumor angiogenesis under high lactic acid conditions in tumors remains poorly understood.
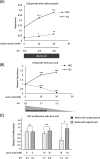
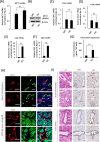
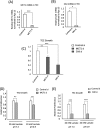
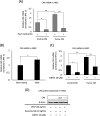
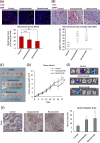
Methodology: The metabolomes of TECs and normal endothelial cells (NECs) were analyzed by capillary electrophoresis time-of-flight mass spectrometry. The expressions of pH regulators in TECs and NECs were determined by quantitative reverse transcription-PCR. Cell proliferation was measured by the MTS assay. Western blotting and ELISA were used to validate monocarboxylate transporter 1 and carbonic anhydrase 2 (CAII) protein expression within the cells, respectively. Human tumor xenograft models were used to access the effect of CA inhibition on tumor angiogenesis. Immunohistochemical staining was used to observe CAII expression, quantify tumor microvasculature, microvessel pericyte coverage, and hypoxia.
Results: The present study shows that, unlike NECs, TECs proliferate in lactic acidic. TECs showed an upregulated CAII expression both in vitro and in vivo. CAII knockdown decreased TEC survival under lactic acidosis and nutrient-replete conditions. Vascular endothelial growth factor A and vascular endothelial growth factor receptor signaling induced CAII expression in NECs. CAII inhibition with acetazolamide minimally reduced tumor angiogenesis in vivo. However, matured blood vessel number increased after acetazolamide treatment, similar to bevacizumab treatment. Additionally, acetazolamide-treated mice showed decreased lung metastasis.
Conclusion: These findings suggest that due to their effect on blood vessel maturity, pH regulators like CAII are promising targets of antiangiogenic therapy. Video Abstract.
Keywords: Angiogenesis; Carbonic anhydrase 2 (CAII); Lactic acidosis; Tumor endothelial cells; pH regulation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
The Epigenetic Profile of Tumor Endothelial Cells. Effects of Combined Therapy with Antiangiogenic and Epigenetic Drugs on Cancer Progression.Int J Mol Sci. 2020 Apr 9;21(7):2606. doi: 10.3390/ijms21072606. Int J Mol Sci. 2020. PMID: 32283668 Free PMC article. Review.
-
A surface proton antenna in carbonic anhydrase II supports lactate transport in cancer cells.Elife. 2018 May 29;7:e35176. doi: 10.7554/eLife.35176. Elife. 2018. PMID: 29809145 Free PMC article.
-
Analysis of the binding moiety mediating the interaction between monocarboxylate transporters and carbonic anhydrase II.J Biol Chem. 2015 Feb 13;290(7):4476-86. doi: 10.1074/jbc.M114.624577. Epub 2015 Jan 5. J Biol Chem. 2015. PMID: 25561737 Free PMC article.
-
Carbonic anhydrase II mediates malignant behavior of pulmonary neuroendocrine tumors.Am J Respir Cell Mol Biol. 2015 Feb;52(2):183-92. doi: 10.1165/rcmb.2014-0054OC. Am J Respir Cell Mol Biol. 2015. PMID: 25019941
-
Contribution of Tumor Endothelial Cells in Cancer Progression.Int J Mol Sci. 2018 Apr 24;19(5):1272. doi: 10.3390/ijms19051272. Int J Mol Sci. 2018. PMID: 29695087 Free PMC article. Review.
Cited by
-
The Epigenetic Profile of Tumor Endothelial Cells. Effects of Combined Therapy with Antiangiogenic and Epigenetic Drugs on Cancer Progression.Int J Mol Sci. 2020 Apr 9;21(7):2606. doi: 10.3390/ijms21072606. Int J Mol Sci. 2020. PMID: 32283668 Free PMC article. Review.
-
Cellular heterogeneity and stem cells of vascular endothelial cells in blood vessel formation and homeostasis: Insights from single-cell RNA sequencing.Front Cell Dev Biol. 2023 Mar 21;11:1146399. doi: 10.3389/fcell.2023.1146399. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37025170 Free PMC article. Review.
-
Regulatory role and therapeutic prospect of lactate modification in cancer.Front Pharmacol. 2025 Feb 17;16:1508552. doi: 10.3389/fphar.2025.1508552. eCollection 2025. Front Pharmacol. 2025. PMID: 40034817 Free PMC article. Review.
-
Angiogenic inhibitor pre-administration improves the therapeutic effects of immunotherapy.Cancer Med. 2023 Apr;12(8):9760-9773. doi: 10.1002/cam4.5696. Epub 2023 Feb 19. Cancer Med. 2023. PMID: 36808261 Free PMC article.
-
[Anti-angiogenesis in Lung Cancer: Current Situation, Progress and Confusion].Zhongguo Fei Ai Za Zhi. 2022 Apr 20;25(4):278-286. doi: 10.3779/j.issn.1009-3419.2022.101.16. Zhongguo Fei Ai Za Zhi. 2022. PMID: 35477192 Free PMC article. Review. Chinese.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

