A Nanobody Against Cytotoxic T-Lymphocyte Associated Antigen-4 Increases the Anti-Tumor Effects of Specific CD8+ T Cells
- PMID: 31847937
- PMCID: PMC9514155
- DOI: 10.1166/jbn.2019.2859
A Nanobody Against Cytotoxic T-Lymphocyte Associated Antigen-4 Increases the Anti-Tumor Effects of Specific CD8+ T Cells
Abstract
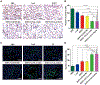
Adoptive cell-based immunotherapy typically utilizes cytotoxic T lymphocytes (CTLs), expanding these cells ex vivo. Such expansion is traditionally accomplished through the use of autologous APCs that are capable of interactions with T cells. However, incidental inhibitory program such as CTLA-4 pathway can impair T cell proliferation. We therefore designed a nanobody which is specific for CTLA-4 (CTLA-4 Nb 16), and we then used this molecule to assess its ability to disrupt CTLA-4 signaling and thereby overcome negative costimulation of T cells. With CTLA-4 Nb16 stimulation, dendritic cell/hepatocellular carcinoma fusion cells (DC/HepG2-FCs) enhanced autologous CD8+ T cell proliferation and production of IFN-γ in vitro, thereby leading to enhanced killing of tumor cells. Using this approach in the context of adoptive CD8+ immunotherapy led to a marked suppression of tumor growth in murine NOD/SCID hepatocarcinoma or breast cancer xenograft models. We also observed significantly increased tumor cell apoptosis, and corresponding increases in murine survival. These findings thus demonstrate that in response to nanobody stimulation, DC/tumor cells-FC-induced specific CTLs exhibit superior anti-tumor efficacy, making this a potentially valuable means of achieving better adoptive immunotherapy outcomes in cancer patients.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Figures



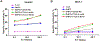


References
-
- Hersey P, and Gallagher S, 2014. Intralesional immunotherapy for melanoma. Journal of surgical oncology, 109(4), pp.320–326. - PubMed
-
- Zhang W, Zhu XD, Sun HC, Xiong YQ, Zhuang PY, Xu HX, and Tang ZY, 2010. Depletion of tumor-associated macrophages enhances the effect of sorafenib in metastatic liver cancer models by antimetastatic and antiangiogenic effects. Clinical Cancer Research, 16(13), pp.3420–3430. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

