Risk of spread in adult-onset isolated focal dystonia: a prospective international cohort study
- PMID: 31848221
- PMCID: PMC7024047
- DOI: 10.1136/jnnp-2019-321794
Risk of spread in adult-onset isolated focal dystonia: a prospective international cohort study
Abstract
Objective: Isolated focal dystonia can spread to muscles beyond the initially affected body region, but risk of spread has not been evaluated in a prospective manner. Furthermore, body regions at risk for spread and the clinical factors associated with spread risk are not well characterised. We sought here to prospectively characterise risk of spread in recently diagnosed adult-onset isolated focal dystonia patients.
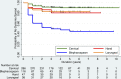
Methods: Patients enrolled in the Dystonia Coalition with isolated dystonia affecting only the neck, upper face, hand or larynx at onset of symptoms were included. Timing of follow-up visits was based on a sliding scale depending on symptom onset and ranged from 1 to 4 years. Descriptive statistics, Kaplan-Meier survival curves and Cox proportional hazard regression models were used to assess clinical characteristics associated with dystonia spread.
Results: 487 enrolled participants (68.3% women; mean age: 55.6±12.2 years) met our inclusion/exclusion criteria. Spread was observed in 50% of blepharospasm, 8% of cervical dystonia, 17% of hand dystonia and 16% of laryngeal dystonia cases. Most common regions for first spread were the oromandibular region (42.2%) and neck (22.4%) for blepharospasm, hand (3.5%) for cervical dystonia and neck for hand (12.8%) and laryngeal (15.8%) dystonia. Increased spread risk was associated with a positive family history (HR=2.18, p=0.012) and self-reported alcohol responsiveness (HR=2.59, p=0.009).
Conclusions: Initial body region affected in isolated focal dystonia has differential risk and patterns of spread. Genetic factors likely influence the risk of spread. These findings can aid clinical prognostication and inform future investigations into potential disease-modifying treatments.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: BDB: The author has received research grant support from the Dana Foundation, NIH (NIH/NCATS Colorado CTSI Grant Number KL2 TR001080), Dystonia Coalition (receives the majority of its support through NIH grant NS065701 from the Office of Rare Diseases Research in the National Center for Advancing Translational Science and National Institute of Neurological Disorders and Stroke), and from Mary Rossick Kern and Jerome H. Kern. The author also is a member of the medical advisory boards for the Benign Essential Blepharospasm Research Foundation and the National Spasmodic Torticollis Association. CLG: The author has received research grant support from the Dystonia Medical Research Foundation. SPR: Has received research grant support from NIH (NCRR/NCATS, UNM CTSC KL2 1TR001448-01 and UL1TR001449) and Dystonia Coalition Projects (NIH/NINDS/ORDR) and has received publishing royalties from Springer. Dr. Pirio Richardson serves on the Scientific Advisory Board for the Benign Essential Blepharospasm Research Foundation. SHS, SAN, JAV, SL, MV, CB and ER: The author has no relevant financial disclosures to report. JJ: Has received a travel grant of the Movement Disorder Society. NB: Has received grant support from DFG (BR4328.2-1, GRK1957), the Collaborative Center for X-linked Dystonia-Parkinsonism and the Else-Kröner Fresenius-Stifung (HA17_2017), has received speaker’s honoraria from Grünenthal, UCB and Teva, and has received non-financial support from Bayer. PA: Has received honoraria from US world Meds, Acadia, and Lundbeck. RLB: Serves as an associate editor for Neurology: Clinical Practice; performs botulinum toxin injections at the University of Rochester (30% effort); serves/has served on scientific advisory board for Allergan, Ipsen, Merz and Revance; receives research support from Vaccinex, Fox Foundation, and Revance; has received research support from NIH (NINDS, ORDR): Dystonia Coalition Projects, Site PI; holds stock options in VisualDx; and has served as an expert witness in legal proceedings including malpractice, not involving commercial entities. AJE: Has received research grants from the NIH, Great Lakes Neurotechnologies and the Michael J Fox Foundation; personal compensation as a consultant/scientific advisory board member for Abbvie, Adamas, Acadia, Acorda, Neuroderm, Impax, Sunovion, Lundbeck, Osmotica Pharmaceutical, and USWorldMeds; publishing royalties from Lippincott Williams & Wilkins, Cambridge University Press, and Springer; and honoraria from USWorldMeds, Lundbeck, Acadia, Sunovion, the American Academy of Neurology, and the Movement Disorders Society. CK: has been supported by the German Research Foundation (FOR 2488) and serves as a medical advisor to Biogen and Centogene. TB: receives funding from the German Research Foundation (FOR 2698), has received honoraria from Merz Pharmaceuticals, Allergan and Ipsen Pharma, and serves as a medical advisor to Merz Pharmaceuticals and Allergan. SGR: Has received research support from the NIH/NINDS, is a reviewer for UpToDate, and has received honoraria from the Movement Disorders Society, and served on a Data Safety Monitoring Board for Enterin. HAJ: Has active or recent grant support from the US government (National Institutes of Health), private philanthropic organizations (the Benign Essential Blepharospasm Research Foundation, Cure Dystonia Now), academically-oriented institutions (the Dystonia Study Group), and industry (Cavion Therapeutics, Ipsen Pharmaceuticals, Retrophin Inc.). Dr. Jinnah has also served on advisory boards or as a consultant for CoA Therapeutics and Retrophin Inc. He has received honoraria or stipends for lectures or administrative work from the American Academy of Neurology, the American Neurological Association, the Dystonia Medical Research Foundation, the International Neurotoxin Society, the International Parkinson’s Disease and Movement Disorders Society, The Parkinson’s Disease Foundation, and Tyler’s Hope for a Cure. Dr. Jinnah serves on the Scientific Advisory Boards for several private foundations including the Benign Essential Blepharospasm Research Foundation, Cure Dystonia Now, the Dystonia Medical Research Foundation, and Tyler's Hope for a Cure. He also is principle investigator for the Dystonia Coalition, which receives the majority of its support through NIH grant TR001456 from the Office of Rare Diseases Research at the National Center for Advancing Translational Sciences, and previously NS065701 from the National Institutes of Neurological Disorders and Stroke. The Dystonia Coalition has received additional material or administrative support from industry sponsors (Allergan Inc. and Merz Pharmaceuticals) as well as private foundations (The American Dystonia Society, Beat Dystonia, The Benign Essential Blepharospasm Foundation, Cure Dystonia Now, Dystonia Europe, Dystonia Inc., Dystonia Ireland, The Dystonia Medical Research Foundation, The Foundation for Dystonia Research, The National Spasmodic Dysphonia Association, and The National Spasmodic Torticollis Association). JSP: Has received research grant support from NIH (NCRR/NCATS, UNM CTSC KL2 1TR001448-01 and UL1TR001449) and Dystonia Coalition Projects (NIH/NINDS/ORDR) and has received publishing royalties from Springer.
Figures
References
-
- Bressman SB. Dystonia genotypes, phenotypes, and classification. Adv Neurol 2004;94:101–7. - PubMed
-
- Defazio G. Epidemiology of Primary and Secondary Dystonia : Stacy MA, Handbook of dystonia. New York, NY: Informa Healthcare USA, Inc, 2007: 11–20.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
