Substrate binding in the processive cellulase Cel7A: Transition state of complexation and roles of conserved tryptophan residues
- PMID: 31848226
- PMCID: PMC7008363
- DOI: 10.1074/jbc.RA119.011420
Substrate binding in the processive cellulase Cel7A: Transition state of complexation and roles of conserved tryptophan residues
Abstract
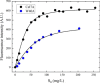
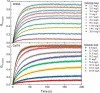
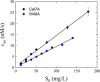
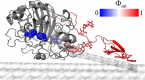
Cellobiohydrolases effectively degrade cellulose and are of biotechnological interest because they can convert lignocellulosic biomass to fermentable sugars. Here, we implemented a fluorescence-based method for real-time measurements of complexation and decomplexation of the processive cellulase Cel7A and its insoluble substrate, cellulose. The method enabled detailed kinetic and thermodynamic analyses of ligand binding in a heterogeneous system. We studied WT Cel7A and several variants in which one or two of four highly conserved Trp residues in the binding tunnel had been replaced with Ala. WT Cel7A had on/off-rate constants of 1 × 105 m-1 s-1 and 5 × 10-3 s-1, respectively, reflecting the slow dynamics of a solid, polymeric ligand. Especially the off-rate constant was many orders of magnitude lower than typical values for small, soluble ligands. Binding rate and strength both were typically lower for the Trp variants, but effects of the substitutions were moderate and sometimes negligible. Hence, we propose that lowering the activation barrier for complexation is not a major driving force for the high conservation of the Trp residues. Using so-called Φ-factor analysis, we analyzed the kinetic and thermodynamic results for the variants. The results of this analysis suggested a transition state for complexation and decomplexation in which the reducing end of the ligand is close to the tunnel entrance (near Trp-40), whereas the rest of the binding tunnel is empty. We propose that this structure defines the highest free-energy barrier of the overall catalytic cycle and hence governs the turnover rate of this industrially important enzyme.
Keywords: Transition state; cellobiohydrolase; cellulase; complex; enzyme kinetics; fluorescence; pre-steady-state kinetics; protein engineering; thermodynamics; tryptophan.
© 2020 Røjel et al.
Conflict of interest statement
T. H. S., A. M. C., and K. B. work at Novozymes, a major enzyme-producing company
Figures


References
-
- Nill J., Karuna N., and Jeoh T. (2018) The impact of kinetic parameters on cellulose hydrolysis rates. Process Biochem. 74, 108–117 10.1016/j.procbio.2018.07.006 - DOI
-
- Payne C. M., Himmel M. E., Crowley M. F., and Beckham G. T. (2011) Decrystallization of oligosaccharides from the cellulose I β surface with molecular simulation. J. Phys. Chem. Lett. 2, 1546–1550 10.1021/jz2005122 - DOI
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

