Characterization of multi-channel intraneural stimulation in transradial amputees
- PMID: 31848384
- PMCID: PMC6917705
- DOI: 10.1038/s41598-019-55591-z
Characterization of multi-channel intraneural stimulation in transradial amputees
Abstract
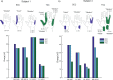
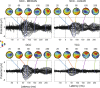
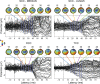
Although peripheral nerve stimulation using intraneural electrodes has been shown to be an effective and reliable solution to restore sensory feedback after hand loss, there have been no reports on the characterization of multi-channel stimulation. A deeper understanding of how the simultaneous stimulation of multiple electrode channels affects the evoked sensations should help in improving the definition of encoding strategies for bidirectional prostheses. We characterized the sensations evoked by simultaneous stimulation of median and ulnar nerves (multi-channel configuration) in four transradial amputees who had been implanted with four TIMEs (Transverse Intrafascicular Multichannel Electrodes). The results were compared with the characterization of single-channel stimulation. The sensations were characterized in terms of location, extent, type, and intensity. Combining two or more single-channel configurations caused a linear combination of the sensation locations and types perceived with such single-channel stimulations. Interestingly, this was also true when two active sites from the same nerve were stimulated. When stimulating in multi-channel configuration, the charge needed from each electrode channel to evoke a sensation was significantly lower than the one needed in single-channel configuration (sensory facilitation). This result was also supported by electroencephalography (EEG) recordings during nerve stimulation. Somatosensory potentials evoked by multi-channel stimulation confirmed that sensations in the amputated hand were perceived by the subjects and that a perceptual sensory facilitation occurred. Our results should help the future development of more efficient bidirectional prostheses by providing guidelines for the development of more complex stimulation approaches to effectively restore multiple sensations at the same time.
Conflict of interest statement
S.R., F.P. and S.M. hold shares of Sensars Neuroprosthetics Sarl, a start-up company dealing with potential commercialization of neurocontrolled artificial limbs. The other authors do not have anything to disclose. All authors have no no-financial interests to declare.
Figures



References
-
- Horch K, Meek S, Taylor TG, Hutchinson DT. Object Discrimination With an Artificial Hand Using Electrical Stimulation of Peripheral Tactile and Proprioceptive Pathways With Intrafascicular Electrodes. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2011;19:483–489. doi: 10.1109/TNSRE.2011.2162635. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

