External quality assessment demonstrates that PD-L1 22C3 and SP263 assays are systematically different
- PMID: 31849189
- PMCID: PMC7164369
- DOI: 10.1002/cjp2.153
External quality assessment demonstrates that PD-L1 22C3 and SP263 assays are systematically different
Abstract
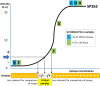
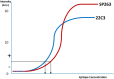
PD-L1 inhibitors are part of first line treatment options for patients with advanced non-small cell lung cancer. PD-L1 immunohistochemistry (IHC) assays act as either a companion or a complementary diagnostic. The purpose of this study is to describe the experience of external quality assurance (EQA) provider UK NEQAS ICC and ISH with the comparison of different PD-L1 assays used in daily practice. Three EQA rounds (pilot, run A and run B) were carried out using formalin fixed paraffin embedded samples with sample sets covering a range of epitope concentrations, including 'critical samples' near to clinical threshold cut-offs. An expert panel (n = 4) evaluated all returned slides simultaneously and independently on a multi-header microscope together with the participants own in-house control material. The tonsil sample was evaluated as 'acceptable' or 'unacceptable', and for the other samples the percentage of PD-L1 stained tumour cells were estimated in predetermined categories (<1%, 1 to <5%, 5 to <10%, 10 to <25%, 25 to <50%, 50 to <80%, 80 to 100%). In the pilot and the two subsequent runs the number of participating laboratories was 43, 69 and 76, respectively. The pass rate for the pilot run was 67%; this increased to 81% at run A and 82% at run B. For two 'critical samples', in runs A and B, 22C3 IHC had significantly higher PD-L1 expression than SP263 IHC (p < 0.001), whilst the PD-L1 scores for the other six samples were similar for all assays. In run A the laboratory developed tests (LDTs) using 22C3 scored lower than the commercial 22C3 tests (p = 0.01). After the initial testing, improvement in performance of PD-L1 IHC is shown for approved and LDT PD-L1 assays. Equivalency of approved PD-L1 22C3 and SP263 assays cannot be assumed as the scores cross the clinically relevant thresholds of 1% and 50% PD-L1 expression.
Keywords: PD-L1; companion diagnostic assays; external quality assessment; immunohistochemistry; non-small cell lung cancer; predictive testing.
© 2019 The Authors. The Journal of Pathology: Clinical Research published by The Pathological Society of Great Britain and Ireland and John Wiley & Sons Ltd.
Figures
References
-
- Lindeman NI, Cagle PT, Aisner DL, et al Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch Pathol Lab Med 2018; 142: 321–346. - PubMed
-
- Planchard D, Popat S, Kerr K, et al Metastatic non‐small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2018; 29: iv192–iv237. - PubMed
-
- Antonia SJ, Villegas A, Daniel D, et al Durvalumab after chemoradiotherapy in stage III non–small‐cell lung cancer. N Engl J Med 2017; 377: 1919–1929. - PubMed
-
- Gandhi L, Rodríguez‐Abreu D, Gadgeel S, et al Pembrolizumab plus chemotherapy in metastatic non–small‐cell lung cancer. N Engl J Med 2018; 378: 2078–2092. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

