Optimization of an in vivo model to study immunity to Plasmodium falciparum pre-erythrocytic stages
- PMID: 31849326
- PMCID: PMC6918627
- DOI: 10.1186/s12936-019-3055-9
Optimization of an in vivo model to study immunity to Plasmodium falciparum pre-erythrocytic stages
Abstract
Background: The circumsporozoite protein (CSP) of Plasmodium is a key surface antigen that induces antibodies and T-cells, conferring immune protection in animal models and humans. However, much of the work on CSP and immunity has been developed based on studies using rodent or non-human primate CSP antigens, which may not be entirely translatable to CSP expressed by human malaria parasites, especially considering the host specificity of the different species.
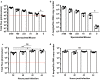
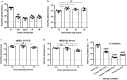
Methods: Using a genetically engineered strain of Plasmodium berghei that expresses luciferase, GFP and the Plasmodium falciparum orthologue of CSP, the effect of laboratory preparation, mosquito treatment and mouse factors on sporozoite infectivity was assessed using an in vivo bioluminescence assay on mice. This assay was compared with a PCR-based protection assay using an already described monoclonal antibody that can provide sterile protection against sporozoite challenge.
Results: Bioluminescence assay demonstrated similar detection levels of the quantity and kinetics of liver-stage infection, compared to PCR-based detection. This assay was used to evaluate treatment of sporozoite and delivery method on mouse infectivity, as well as the effects of age, sex and strain of mice. Finally, this assay was used to test the protective capacity of monoclonal antibody AB317; results strongly recapitulate the findings of previous work on this antibody.
Conclusions: The PbGFP-Luc line and in vivo bioluminescence imaging provide highly sensitive read-outs of liver-stage infection in mice, and this method can be useful to reliably evaluate potency of pre-erythrocytic interventions.
Keywords: Bioluminescence; Malaria; Plasmodium falciparum; Transgenic parasite; Vaccine.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Espinosa DA, Christensen D, Munoz C, Singh S, Locke E, Andersen P, et al. Robust antibody and CD8(+) T-cell responses induced by P. falciparum CSP adsorbed to cationic liposomal adjuvant CAF09 confer sterilizing immunity against experimental rodent malaria infection. NPJ Vaccines. 2017;2:e10. doi: 10.1038/s41541-017-0011-y. - DOI - PMC - PubMed
-
- Gimenez AM, Lima LC, Francoso KS, Denapoli PMA, Panatieri R, Bargieri DY, et al. Vaccine containing the three allelic variants of the Plasmodium vivax circumsporozoite antigen induces protection in mice after challenge with a transgenic rodent malaria parasite. Front Immunol. 2017;8:1275. doi: 10.3389/fimmu.2017.01275. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

