Interstitial Lung Disease in Systemic Sclerosis: Focus on Early Detection and Intervention
- PMID: 31849543
- PMCID: PMC6910104
- DOI: 10.2147/OARRR.S226695
Interstitial Lung Disease in Systemic Sclerosis: Focus on Early Detection and Intervention
Abstract
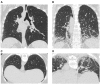
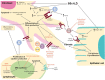
Systemic sclerosis (SSc) is a progressive and often devastating disease characterized by autoimmune dysfunction, vasculopathy, and fibrosis. Interstitial lung disease (ILD) is identified in the majority of patients with SSc and is the leading cause of SSc-related mortality. Although clinical manifestations and ILD severity vary among patients, lung function typically declines to the greatest extent during the first 3-4 years after disease onset. We aim to provide an overview of SSc-associated ILD (SSc-ILD) with a focus on current and emerging tools for early diagnosis of ILD and current and novel treatments under investigation. Early detection of ILD provides the opportunity for early therapeutic intervention, which could improve patient outcomes. Thoracic high-resolution computed tomography is the most effective method of identifying ILD in patients with SSc; it enables detection of mild lung abnormalities and plays an important role in monitoring disease progression. Cyclophosphamide and mycophenolate mofetil are the most commonly prescribed treatments for SSc-ILD. Recently, nintedanib (an antifibrotic) was approved by the Food and Drug Administration for patients with SSc-ILD; it is indicated for slowing the rate of decline in pulmonary function. However, there is a need for additional effective and well-tolerated disease-modifying therapy. Ongoing studies are evaluating other antifibrotics and novel agents. We envision that early detection of lung involvement, combined with the emergence and integration of novel therapies, will lead to improved outcomes in patients with SSc-ILD.
Keywords: disease progression; early diagnosis; interstitial lung diseases; systemic sclerosis; treatment outcome.
© 2019 Fischer et al.
Conflict of interest statement
AF reports receiving grants and personal fees from Boehringer Ingelheim Pharmaceuticals, Inc. during the development of the submitted work and personal fees from Genentech Inc./F. Hoffmann-La Roche Ltd, Pfizer Inc. and Bristol-Myers Squibb outside the submitted work. AF is currently an employee of Bristol-Myers Squibb Company, Princeton, NJ. NMP and ERV both report receiving personal fees from Boehringer Ingelheim Pharmaceuticals, Inc. outside the submitted work. The authors report no other conflicts of interest in this work.
Figures
References
-
- Hinchcliff M, Varga J. Systemic sclerosis/scleroderma: a treatable multisystem disease. Am Fam Physician. 2008;78(8):961–968. - PubMed

