Insulin Signaling as a Key Moderator in Myotonic Dystrophy Type 1
- PMID: 31849810
- PMCID: PMC6901991
- DOI: 10.3389/fneur.2019.01229
Insulin Signaling as a Key Moderator in Myotonic Dystrophy Type 1
Abstract
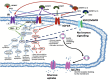
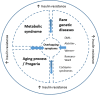
Myotonic dystrophy type 1 (DM1) is an autosomal dominant genetic disease characterized by multi-system involvement. Affected organ system includes skeletal muscle, heart, gastro-intestinal system and the brain. In this review, we evaluate the evidence for alterations in insulin signaling and their relation to clinical DM1 features. We start by summarizing the molecular pathophysiology of DM1. Next, an overview of normal insulin signaling physiology is given, and evidence for alterations herein in DM1 is presented. Clinically, evidence for involvement of insulin signaling pathways in DM1 is based on the increased incidence of insulin resistance seen in clinical practice and recent trial evidence of beneficial effects of metformin on muscle function. Indirectly, further support may be derived from certain CNS derived symptoms characteristic of DM1, such as obsessive-compulsive behavior features, for which links with altered insulin signaling has been demonstrated in other diseases. At the basic scientific level, several pathophysiological mechanisms that operate in DM1 may compromise normal insulin signaling physiology. The evidence presented here reflects the importance of insulin signaling in relation to clinical features of DM1 and justifies further basic scientific and clinical, therapeutically oriented research.
Keywords: behavioral flexibility; diabetes type 2; insulin; insulin resistance; insulin-like growth factor 1 (IGF1); metformin; myotonic dystrophy (DM1); obsessive–compulsive disorder.
Copyright © 2019 Nieuwenhuis, Okkersen, Widomska, Blom, 't Hoen, van Engelen and Glennon.
Figures

Similar articles
-
Metabolic Alterations in Myotonic Dystrophy Type 1 and Their Correlation with Lipin.Int J Environ Res Public Health. 2021 Feb 12;18(4):1794. doi: 10.3390/ijerph18041794. Int J Environ Res Public Health. 2021. PMID: 33673200 Free PMC article. Review.
-
Chronic exercise mitigates disease mechanisms and improves muscle function in myotonic dystrophy type 1 mice.J Physiol. 2019 Mar;597(5):1361-1381. doi: 10.1113/JP277123. Epub 2019 Jan 30. J Physiol. 2019. PMID: 30628727 Free PMC article.
-
Immortalized human myotonic dystrophy muscle cell lines to assess therapeutic compounds.Dis Model Mech. 2017 Apr 1;10(4):487-497. doi: 10.1242/dmm.027367. Epub 2017 Feb 10. Dis Model Mech. 2017. PMID: 28188264 Free PMC article.
-
[Glucose intolerance in myotonic dystrophy type 1].Rinsho Shinkeigaku. 2012;52(11):1259-60. doi: 10.5692/clinicalneurol.52.1259. Rinsho Shinkeigaku. 2012. PMID: 23196582 Japanese.
-
Myotonic dystrophy: emerging mechanisms for DM1 and DM2.Biochim Biophys Acta. 2007 Feb;1772(2):195-204. doi: 10.1016/j.bbadis.2006.05.013. Epub 2006 Jun 20. Biochim Biophys Acta. 2007. PMID: 16876389 Review.
Cited by
-
Metabolic Alterations in Myotonic Dystrophy Type 1 and Their Correlation with Lipin.Int J Environ Res Public Health. 2021 Feb 12;18(4):1794. doi: 10.3390/ijerph18041794. Int J Environ Res Public Health. 2021. PMID: 33673200 Free PMC article. Review.
-
The Splice Index as a prognostic biomarker of strength and function in myotonic dystrophy type 1.J Clin Invest. 2025 Jan 7;135(4):e185426. doi: 10.1172/JCI185426. J Clin Invest. 2025. PMID: 39836447 Free PMC article.
-
Targeting Myotonic Dystrophy Type 1 with Metformin.Int J Mol Sci. 2022 Mar 7;23(5):2901. doi: 10.3390/ijms23052901. Int J Mol Sci. 2022. PMID: 35270043 Free PMC article. Review.
-
A case of early onset diabetes with myotonic dystrophy type 1.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2023 Jun 28;48(6):930-934. doi: 10.11817/j.issn.1672-7347.2023.220599. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2023. PMID: 37587079 Free PMC article. Chinese, English.
-
Automatic Text-Mining Approach to Identify Molecular Target Candidates Associated with Metabolic Processes for Myotonic Dystrophy Type 1.Int J Environ Res Public Health. 2023 Jan 27;20(3):2283. doi: 10.3390/ijerph20032283. Int J Environ Res Public Health. 2023. PMID: 36767649 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

