Treatment of Hypothyroid Patients With L-Thyroxine (L-T4) Plus Triiodothyronine Sulfate (T3S). A Phase II, Open-Label, Single Center, Parallel Groups Study on Therapeutic Efficacy and Tolerability
- PMID: 31849843
- PMCID: PMC6896827
- DOI: 10.3389/fendo.2019.00826
Treatment of Hypothyroid Patients With L-Thyroxine (L-T4) Plus Triiodothyronine Sulfate (T3S). A Phase II, Open-Label, Single Center, Parallel Groups Study on Therapeutic Efficacy and Tolerability
Abstract
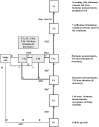
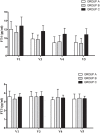
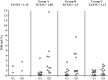
Sodium salt of levothyroxine (L-T4) is the treatment of choice of hypothyroidism. Yet, L-T4 monotherapy produces supoptimal 3,5,3'-triiodothyronine (T3)/T4 ratio in serum, as compared to normal subjects, and a minority of hypothyroid individuals on L-T4 complain for an incomplete well-being. Orally administered 3,5,3'-triiodothyronine sulfate (T3S) can be converted to T3 in humans, resulting in steady-state serum T3 concentrations for up to 48 h. In this study (EudraCT number 2010-018663-42), 36 thyroidectomized hypothyroid patients receiving 100 (group A), 125 (group B), or 150 μg (group C) L-T4 were enrolled in a 75 days study in which 25 μg L-T4 were replaced by 40 μg of T3S. A significant, progressive reduction in mean FT4 values was observed, being the largest in the group A and the smallest in group C, while no relevant variations in FT3 and total T3 serum values were observed in the three groups. TSH serum levels increased in all groups, the highest value being observed in group A. Lipid parameters did not show clinically significant changes in all groups. No T3S-related changes in the safety laboratory tests were recorded. No adverse event was judged as related to experimental treatment, and no patient discontinued the treatment. Twelve patients judged the L-T4+T3S treatment better than L-T4 alone, while no patient reported a preference for L-T4 over the combined treatment. In conclusion, the results of this study indicate that a combination of L-T4+T3S in hypothyroid subjects may allow mainteinance of normal levels of serum T3, with restoration of a physiological FT4/FT3 ratio and no appearance of adverse events. Further studies are required to verify whether the LT4+T3S chronic combined treatment of hypothyroidism is able to produce additional benefits over L-T4 monotherapy.
Keywords: 3,5,3′-triiodothyronine sulfate; L-Thyroxine; hypothyroidism; substitutive therapy; thyroid hormone metabolism.
Copyright © 2019 Santini, Ceccarini, Pelosini, Giannetti, Ricco, Querci, Grossi, Saponati and Vitti.
Figures

References
-
- Santini F, Pinchera A. Causes and laboratory investigation of hypothyroidism. In: Wass JAH, Stewart PM. editors. Oxford Textbook of Endocrinology and Diabetes, 2nd Edn. Oxford: Oxford University Press; (2011). p. 530–6. 10.1093/med/9780199235292.003.3243 - DOI
-
- Peeters RP, Visser TJ. Metabolism of thyroid hormone. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, Hershman JM, et al. editors. Endotext (South Dartmouth, MA: MDText.com, Inc.) (2017).
LinkOut - more resources
Full Text Sources
Other Literature Sources

