Mucosal Immunity and the FOXO1 Transcription Factors
- PMID: 31849924
- PMCID: PMC6896163
- DOI: 10.3389/fimmu.2019.02530
Mucosal Immunity and the FOXO1 Transcription Factors
Abstract
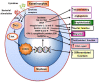
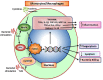
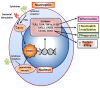
FOXO1 transcription factors affect a number of cell types that are important in the host response. Cell types whose functions are modulated by FOXO1 include keratinocytes in the skin and mucosal dermis, neutrophils and macrophages, dendritic cells, Tregs and B-cells. FOXO1 is activated by bacterial or cytokine stimulation. Its translocation to the nucleus and binding to promoter regions of genes that have FOXO response elements is stimulated by the MAP kinase pathway and inhibited by the PI3 kinase/AKT pathway. Downstream gene targets of FOXO1 include pro-inflammatory signaling molecules (TLR2, TLR4, IL-1β, and TNF-α), wound healing factors (TGF-β, VEGF, and CTGF) adhesion molecules (integrins-β1, -β3, -β6, αvβ3, CD11b, CD18, and ICAM-1), chemokine receptors (CCR7 and CXCR2), B cell regulators (APRIL and BLYS), T-regulatory modulators (Foxp3 and CTLA-4), antioxidants (GPX-2 and cytoglobin), and DNA repair enzymes (GADD45α). Each of the above cell types are found in oral mucosa and modulated by bacteria or an inflammatory microenvironment. FOXO1 contributes to the regulation of these cells, which collectively maintain and repair the epithelial barrier, formation and activation of Tregs that are needed to resolve inflammation, mobilization, infiltration, and activation of anti-bacterial defenses in neutrophils, and the homing of dendritic cells to lymph nodes to induce T-cell and B-cell responses. The goal of the manuscript is to review how the transcription factor, FOXO1, contributes to the activation and regulation of key leukocytes needed to maintain homeostasis and respond to bacterial challenge in oral mucosal tissues. Examples are given with an emphasis on lineage specific deletion of Foxo1 to explore the impact of FOXO1 on cell behavior, inflammation and susceptibility to infection.
Keywords: bacteria; bone loss; forkhead; gingiva; immune; mucosa; periodontal disease; periodontitis.
Copyright © 2019 Graves and Milovanova.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

