Elucidating the Immune Evasion Mechanisms of Borrelia mayonii, the Causative Agent of Lyme Disease
- PMID: 31849943
- PMCID: PMC6902028
- DOI: 10.3389/fimmu.2019.02722
Elucidating the Immune Evasion Mechanisms of Borrelia mayonii, the Causative Agent of Lyme Disease
Abstract
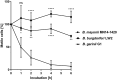
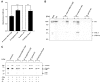
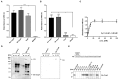
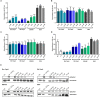
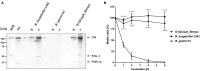
Borrelia (B.) mayonii sp. nov. has recently been reported as a novel human pathogenic spirochete causing Lyme disease (LD) in North America. Previous data reveal a higher spirochaetemia in the blood compared to patients infected by LD spirochetes belonging to the B. burgdorferi sensu lato complex, suggesting that this novel genospecies must exploit strategies to overcome innate immunity, in particular complement. To elucidate the molecular mechanisms of immune evasion, we utilized various methodologies to phenotypically characterize B. mayonii and to identify determinants involved in the interaction with complement. Employing serum bactericidal assays, we demonstrated that B. mayonii resists complement-mediated killing. To further elucidate the role of the key regulators of the alternative pathway (AP), factor H (FH), and FH-like protein 1 (FHL-1) in immune evasion of B. mayonii, serum adsorption experiments were conducted. The data revealed that viable spirochetes recruit both regulators from human serum and FH retained its factor I-mediated C3b-inactivating activity when bound to the bacterial cells. In addition, two prominent FH-binding proteins of approximately 30 and 18 kDa were detected in B. mayonii strain MN14-1420. Bioinformatics identified a gene, exhibiting 60% identity at the DNA level to the cspA encoding gene of B. burgdorferi. Following PCR amplification, the gene product was produced as a His-tagged protein. The CspA-orthologous protein of B. mayonii interacted with FH and FHL-1, and both bound regulators promoted inactivation of C3b in the presence of factor I. Additionally, the CspA ortholog counteracted complement activation by inhibiting the alternative and terminal but not the classical and Lectin pathways, respectively. Increasing concentrations of CspA of B. mayonii also strongly affected C9 polymerization, terminating the formation of the membrane attack complex. To assess the role of CspA of B. mayonii in facilitating serum resistance, a gain-of-function strain was generated, harboring a shuttle vector allowing expression of the CspA encoding gene under its native promotor. Spirochetes producing the native protein on the cell surface overcame complement-mediated killing, indicating that CspA facilitates serum resistance of B. mayonii. In conclusion, here we describe the molecular mechanism utilized by B. mayonii to resists complement-mediated killing by capturing human immune regulators.
Keywords: borrelia; borrelia mayonii; complement; host cell interaction; immune evasion; innate immunity; lyme disease; spirochetes.
Copyright © 2019 Walter, Sürth, Röttgerding, Zipfel, Fritz-Wolf and Kraiczy.
Figures



References
-
- Pritt BS, Mead PS, Johnson DKH, Neitzel DF, Respicio-Kingry LB, Davis JP, et al. . Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dise. (2016) 16:556–64. 10.1016/S1473-3099(15)00464-8 - DOI - PMC - PubMed
-
- Pritt BS, Respicio-Kingry LB, Sloan LM, Schriefer ME, Replogle AJ, Bjork J, et al. . Borrelia mayonii sp. nov., a member of the Borrelia burgdorferi sensu lato complex, detected in patients and ticks in the upper midwestern United States. Int J Syst Evol Microbiol. (2016) 66:4878–80. 10.1099/ijsem.0.001445 - DOI - PMC - PubMed
-
- Eisen L, Breuner NE, Hojgaard A, Hoxmeier JC, Pilgard MA, Replogle AJ, et al. . Comparison of vector efficiency of Ixodes scapularis (Acari: Ixodidae) from the northeast and upper midwest of the United States for the Lyme disease spirochete Borrelia mayonii. J Med Entomol. (2017) 54:239–42. 10.1093/jme/tjw160 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

