Innate Immune Responses in the Outcome of Haploidentical Hematopoietic Stem Cell Transplantation to Cure Hematologic Malignancies
- PMID: 31849972
- PMCID: PMC6892976
- DOI: 10.3389/fimmu.2019.02794
Innate Immune Responses in the Outcome of Haploidentical Hematopoietic Stem Cell Transplantation to Cure Hematologic Malignancies
Abstract
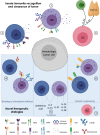
In the context of allogeneic transplant platforms, human leukocyte antigen (HLA)-haploidentical hematopoietic stem cell transplantation (haplo-HSCT) represents one of the latest and most promising curative strategies for patients affected by high-risk hematologic malignancies. Indeed, this platform ensures a suitable stem cell source immediately available for virtually any patents in need. Moreover, the establishment in recipients of a state of immunologic tolerance toward grafted hematopoietic stem cells (HSCs) remarkably improves the clinical outcome of this transplant procedure in terms of overall and disease free survival. However, the HLA-mismatch between donors and recipients has not been yet fully exploited in order to optimize the Graft vs. Leukemia effect. Furthermore, the efficacy of haplo-HSCT is currently hampered by several life-threatening side effects including the onset of Graft vs. Host Disease (GvHD) and the occurrence of opportunistic viral infections. In this context, the quality and the kinetic of the immune cell reconstitution (IR) certainly play a major role and several experimental efforts have been greatly endorsed to better understand and accelerate the post-transplant recovery of a fully competent immune system in haplo-HSCT. In particular, the IR of innate immune system is receiving a growing interest, as it recovers much earlier than T and B cells and it is able to rapidly exert protective effects against both tumor relapses, GvHD and the onset of life-threatening opportunistic infections. Herein, we review our current knowledge in regard to the kinetic and clinical impact of Natural Killer (NK), γδ and Innate lymphoid cells (ILCs) IRs in both allogeneic and haplo-HSCT. The present paper also provides an overview of those new therapeutic strategies currently being implemented to boost the alloreactivity of the above-mentioned innate immune effectors in order to ameliorate the prognosis of patients affected by hematologic malignancies and undergone transplant procedures.
Keywords: alloreactivity; haploidentical hematopoietic stem cell transplantation; immune-reconstitution; innate lymphocytes; innate lymphoid cells; natural killer cells; γδ T cells.
Copyright © 2019 Zaghi, Calvi, Di Vito and Mavilio.
Figures
References
-
- Esiashvili N, Pulsipher MA. Hematopoietic stem cell transplantation. In: Merchant TE, Kortmann RD, editors. Pediatric Radiation Oncology. Cham: Springer International Publishing; (2018). p. 301–11.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

