A New Era of Prostate Cancer Precision Medicine
- PMID: 31850193
- PMCID: PMC6901987
- DOI: 10.3389/fonc.2019.01263
A New Era of Prostate Cancer Precision Medicine
Abstract
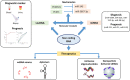
Prostate cancer is the second most common male cancer affecting Western society. Despite substantial advances in the exploration of prostate cancer biomarkers and treatment strategies, men are over diagnosed with inert prostate cancer, while there is also a substantial mortality from the invasive disease. Precision medicine is the management of treatment profiles across different cancers predicting therapies for individual cancer patients. With strategies including individual genomic profiling and targeting specific cancer pathways, precision medicine for prostate cancer has the potential to impose changes in clinical practices. Some of the recent advances in prostate cancer precision medicine comprise targeting gene fusions, genome editing tools, non-coding RNA biomarkers, and the promise of liquid tumor profiling. In this review, we will discuss these recent scientific advances to scale up these approaches and endeavors to overcome clinical barriers for prostate cancer precision medicine.
Keywords: biomarkers; gene fusion; genome editing; liquid biopsy; non-coding genome; precision medicine; prostate cancer; proteomic technologies.
Copyright © 2019 Malik, Srinivasan and Batra.
Figures


References
-
- Olivier C, Bernard M. Biomarkers of aggressiveness in prostate cancer. In: Spiess PE, editor. Prostate Cancer - Diagnostic and Therapeutic Advances. Rijeka: InTech; (2011). p. 3–20. 10.5772/25310 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

