ZBP1 and TAK1: Master Regulators of NLRP3 Inflammasome/Pyroptosis, Apoptosis, and Necroptosis (PAN-optosis)
- PMID: 31850239
- PMCID: PMC6902032
- DOI: 10.3389/fcimb.2019.00406
ZBP1 and TAK1: Master Regulators of NLRP3 Inflammasome/Pyroptosis, Apoptosis, and Necroptosis (PAN-optosis)
Abstract
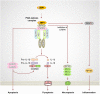
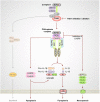
Cell death is central to development, organismal homeostasis, and immune responses. The cell death field has experienced tremendous progress by delineating the molecular programs specific to each of the apoptotic and inflammatory cell death pathways. Moreover, the discovery of the inflammasomes and pyroptosis and necroptosis pathway regulators have provided the genetic basis for the programmed inflammatory cell death pathways. Earlier research highlighted the unique regulation of each of these pathways, but emerging studies discovered co-regulation and crosstalk between these seemingly different cell death complexes. The progress in this area has led to an idea that master regulators play central roles in orchestrating multiple cell death pathways. Here, we provide a brief review of the master regulators, the innate immune sensor ZBP1 and the essential cell survival kinase TAK1, that play vital roles in the regulation of RIPK1/RIPK3-FADD-caspase-8 cell death complex assembly and its versatility in executing Pyroptosis, Apoptosis, and Necroptosis, which we dubbed here as PAN-optosis. Furthermore, we discuss the implications and therapeutic potential of targeting these master regulators in health and disease.
One sentence summary: ZBP1 and TAK1 regulate PAN-optosis.
Keywords: MLKL; TLR priming; caspase-1; gasdermin D; infection; inflammasome; inflammation; innate immunity.
Copyright © 2019 Malireddi, Kesavardhana and Kanneganti.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

