Bacterial Biofilm Eradication Agents: A Current Review
- PMID: 31850313
- PMCID: PMC6893625
- DOI: 10.3389/fchem.2019.00824
Bacterial Biofilm Eradication Agents: A Current Review
Abstract
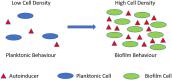
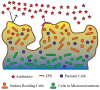
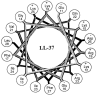
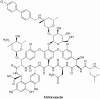
Most free-living bacteria can attach to surfaces and aggregate to grow into multicellular communities encased in extracellular polymeric substances called biofilms. Biofilms are recalcitrant to antibiotic therapy and a major cause of persistent and recurrent infections by clinically important pathogens worldwide (e.g., Pseudomonas aeruginosa, Escherichia coli, and Staphylococcus aureus). Currently, most biofilm remediation strategies involve the development of biofilm-inhibition agents, aimed at preventing the early stages of biofilm formation, or biofilm-dispersal agents, aimed at disrupting the biofilm cell community. While both strategies offer some clinical promise, neither represents a direct treatment and eradication strategy for established biofilms. Consequently, the discovery and development of biofilm eradication agents as comprehensive, stand-alone biofilm treatment options has become a fundamental area of research. Here we review our current understanding of biofilm antibiotic tolerance mechanisms and provide an overview of biofilm remediation strategies, focusing primarily on the most promising biofilm eradication agents and approaches. Many of these offer exciting prospects for the future of biofilm therapeutics for a large number of infections that are currently refractory to conventional antibiotics.
Keywords: antibiotics; bacteria; biofilm; biofilm antibiotic tolerance; biofilm eradication agent; infection; resistance.
Copyright © 2019 Verderosa, Totsika and Fairfull-Smith.
Figures



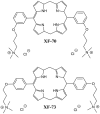
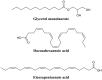
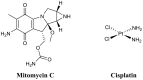
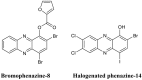
References
-
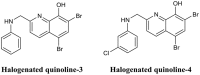
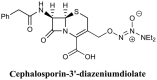
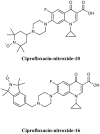
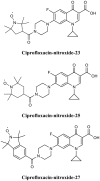
- Abouelhassan Y., Garrison A. T., Burch G. M., Wong W., Norwood V. M., Huigens R. W., III. (2014). Discovery of quinoline small molecules with potent dispersal activity against methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis biofilms using a scaffold hopping strategy. Bioorg. Med. Chem. Lett. 24, 5076–5080. 10.1016/j.bmcl.2014.09.009 - DOI - PubMed
-
- Allen N. E. (2010). From vancomycin to oritavancin: the discovery and development of a novel lipoglycopeptide antibiotic. Antiinfect. Agents Med. Chem. 9, 23–47. 10.2174/187152110790886745 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

