Dual-Venc acquisition for 4D flow MRI in aortic stenosis with spiral readouts
- PMID: 31850597
- PMCID: PMC7299789
- DOI: 10.1002/jmri.27004
Dual-Venc acquisition for 4D flow MRI in aortic stenosis with spiral readouts
Abstract
Background: Single Venc 4D flow MRI with Cartesian readout is hampered by poor velocity resolution and noise when imaging during diastole. Dual Venc acquisitions typically require the acquisition of two distinct datasets, which leads to longer scan times.
Purpose/hypothesis: To design and develop a 4D Spiral Dual Venc sequence. The sequence allows for separate systolic and diastolic Venc s as part of a single acquisition with a prescribed switch time. The implemented sequence was hypothesized to be comparable to Cartesian 4D flow, but with increased velocity resolution in the diastolic phase and with better scan efficiency and reduced noise.
Study type: Prospective.
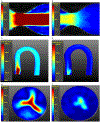
Population: The studied populations were two phantoms-a straight pipe with a stenotic narrowing and a phantom of the aortic arch which included a calcific polymeric valve-under both steady and pulsatile flows, six healthy volunteers, and eight patients with severe aortic stenosis (AS).
Field strength/sequence: 1.5T, Dual Venc 4D flow with spiral readouts.
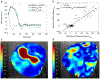
Assessment: Data from the proposed sequence were compared with data from 4D Cartesian Dual Venc and Single Venc acquisitions. Noise was assessed from the acquired velocity data with the pump turned off and by varying Venc . Steady acquisitions were compared to the proximal slice of the lowest Single Venc acquisition.
Statistical tests: Steady flows were compared using relative-root-mean-squared-error (RRMSE). For in vivo flows and pulsatile in vitro flows, net flow for corresponding timepoints were compared with the Pearson correlation test (P < 0.01).
Results: For steady flows, RRMSEs for Single Venc s ranged from 17.6% to 19.4%, and 9.6% to 16.5% for Dual Venc s. The net flow correlation coefficient for the aortic arch phantom was 0.975, and 0.995 for the stenotic phantom. Normal volunteer and patient comparisons yielded a correlation of 0.970 and 0.952, respectively. in vitro and in vivo pulsatile flow waveforms closely matched.
Data conclusion: The Dual Venc offers improved noise properties and velocity resolution, while the spiral trajectory offers a scan efficient acquisition with short echo time yielding reduced flow artifacts.
Level of evidence: 2 Technical Efficacy Stage: 1 J. Magn. Reson. Imaging 2020;52:117-128.
Keywords: 4D flow MRI; Dual Venc; non-Cartesian trajectory; phase contrast MRI; spiral acquisition; stenotic flow.
© 2019 International Society for Magnetic Resonance in Medicine.
Figures
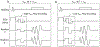




References
-
- Pellikka PA, Sarano ME, Nishimura RA, et al. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation 2005;111:3290–3295. - PubMed
-
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:e57–e185. - PubMed
-
- Bonow RO, Carabello BA, Chatterjee K, et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease) Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008;52:e1–e142. - PubMed
-
- Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med 1999;341:142–147. - PubMed
-
- Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease fn1. J Am Coll Cardiol 1997;29:630–634. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

