Efficacy and safety of pharmacotherapies for smoking cessation in anxiety disorders: Subgroup analysis of the randomized, active- and placebo-controlled EAGLES trial
- PMID: 31850603
- PMCID: PMC7064930
- DOI: 10.1002/da.22982
Efficacy and safety of pharmacotherapies for smoking cessation in anxiety disorders: Subgroup analysis of the randomized, active- and placebo-controlled EAGLES trial
Abstract
Background: Smoking rates are high in adults with anxiety disorders (ADs), yet little is known about the safety and efficacy of smoking-cessation pharmacotherapies in this group.
Methods: Post hoc analyses in 712 smokers with AD (posttraumatic stress disorder [PTSD], n = 192; generalized anxiety disorder [GAD], n = 243; panic disorder [PD], n = 277) and in a nonpsychiatric cohort (NPC; n = 4,028). Participants were randomly assigned to varenicline, bupropion, nicotine-replacement therapy (NRT), or placebo plus weekly smoking-cessation counseling for 12 weeks, with 12 weeks follow-up. General linear models were used to test the effects of treatment group, cohort, and their interaction on neuropsychiatric adverse events (NPSAEs), and continuous abstinence weeks 9-12 (treatment) and 9-24 (follow-up).
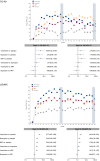
Results: NPSAE incidence for PTSD (6.9%), GAD (5.4%), and PD (6.2%) was higher versus NPC (2.1%), regardless of treatment. Across all treatments, smokers with PTSD (odds ratio [OR] = 0.58), GAD (OR = 0.72), and PD (OR = 0.53) had lower continuous abstinence rates weeks 9-12 (CAR9-12) versus NPC. Varenicline demonstrated superior efficacy to placebo in smokers with GAD and PD, respectively (OR = 4.53; 95% confidence interval [CI] = 1.20-17.10; and OR = 8.49; 95% CI = 1.57-45.78); NRT was superior to placebo in smokers with PD (OR = 7.42; 95% CI = 1.37-40.35). While there was no statistically significant effect of any treatment on CAR9-12 for smokers with PTSD, varenicline improved 7-day point prevalence abstinence at end of treatment in this subcohort.
Conclusion: Individuals with ADs were more likely than those without psychiatric illness to experience moderate to severe NPSAEs during smoking-cessation attempts, regardless of treatment. While the study was not powered to evaluate abstinence outcomes with these subgroups of smokers with ADs, varenicline provided significant benefit for cessation in those with GAD and PD, while NRT provided significant benefit for those with PD.
Trial registration: ClinicalTrials.gov NCT01456936.
Keywords: GAD/generalized anxiety disorder; PTSD/posttraumatic stress disorder; anxiety/anxiety disorders; panic disorder; smoking.
© 2019 The Authors. Depression and Anxiety Published by Wiley Periodicals, Inc.
Conflict of interest statement
C. R. A. and J. L. H. report no conflicts of interest. A. E. E. reports research grants to her institution from Forum Pharmaceuticals and Pfizer and personal fees for consultation or advisory board services from Charles River Analytics, Karuna Pharmaceuticals, Alkermes, Pfizer, and Reckitt Benck iser. A. E. E.'s writing of the manuscript was supported by a National Institute on Drug Abuse Career Award in Patient‐Oriented Research, K24 DA030443. R. M. A. reports his university receiving grants from Alkermes and Pfizer and providing consulting and/or advisory board services to Arena Pharmaceuticals, Cerecor, Pfizer, and US WorldMeds. R. M. A.'s writing of this manuscript was supported, in part, by National Institute on Alcohol Abuse and Alcoholism Grant #s U10 AA008401 and 1R44AA024643, and NIDA Grant # UO1 DA041731. C. R., D. L., and T. M. are employees and stockholders of Pfizer. The opinions expressed in this article are those of the authors and do not necessarily reflect the views of their employers.
Figures



References
-
- American Psychiatric Association . (2000). Diagnostic and statistical manual of mental disorders (4th ed.). Arlington, VA: American Psychiatric Association.
-
- Anthenelli, R. M. , Benowitz, N. L. , West, R., St , Aubin, L. , McRae, T. , Lawrence, D. , … Evins, A. E. (2016). Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): A double‐blind, randomised, placebo‐controlled clinical trial. Lancet, 387(10037), 2507–2520. 10.1016/S0140-6736(16)30272-0 - DOI - PubMed
-
- Anthenelli, R. M. , Gaffney, M. , Benowitz, N. L. , West, R. , McRae, T. , Russ, C. , … Evins, A. E. (2019). Predictors of neuropsychiatric adverse events with smoking cessation medications in the randomized controlled EAGLES trial. Journal of General Internal Medicine, 34, 862–870. 10.1007/s11606-019-04858-2 - DOI - PMC - PubMed
-
- Anthenelli, R. M. , Morris, C. , Ramey, T. S. , Dubrava, S. J. , Tsilkos, K. , Russ, C. , & Yunis, C. (2013). Effects of varenicline on smoking cessation in adults with stably treated current or past major depression: A randomized trial. Annals of Internal Medicine, 159(6), 390–400. 10.7326/0003-4819-159-6-201309170-00005 - DOI - PubMed

