Role of inflammasomes in the pathogenesis of periodontal disease and therapeutics
- PMID: 31850638
- PMCID: PMC6927484
- DOI: 10.1111/prd.12269
Role of inflammasomes in the pathogenesis of periodontal disease and therapeutics
Abstract
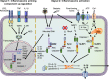
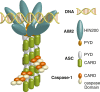
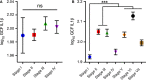
Inflammasomes are a group of multimolecular intracellular complexes assembled around several innate immune proteins. Recognition of a diverse range of microbial, stress and damage signals by inflammasomes results in direct activation of caspase-1, which subsequently induces the only known form of secretion of active interleukin-1β and interleukin-18. Although the importance of interleukin-1β in the periodontium is not questioned, the impact of inflammasomes in periodontal disease and its potential for therapeutics in periodontology is still in its very early stages. Increasing evidence in preclinical models and human data strongly implicate the involvement of inflammasomes in a number of inflammatory, autoinflammatory and autoimmune disorders. Here we review: (a) the currently known inflammasome functions, (b) clinical/preclinical data supporting inflammasome involvement in the context of periodontal and comorbid diseases and (c) potential therapies targeting inflammasomes. To clarify further the inflammasome involvement in periodontitis, we present analyses of data from a large clinical study (n = 5809) that measured the gingival crevicular fluid-interleukin-1β and grouped the participants based on current periodontal disease classifications. We review data on 4910 European-Americans that correlate 16 polymorphisms in the interleukin-1B region with high gingival crevicular fluid-interleukin-1β levels. We show that inflammasome components are increased in diseased periodontal tissues and that the caspase-1 inhibitor, VX-765, inhibits ~50% of alveolar bone loss in experimental periodontitis. The literature review further supports that although patients clinically present with the same phenotype, the disease that develops probably has different underlying biological pathways. The current data indicate that inflammasomes have a role in periodontal disease pathogenesis. Understanding the contribution of different inflammasomes to disease development and distinct patient susceptibility will probably translate into improved, personalized therapies.
© 2019 The Authors. Periodontology 2000 Published by John Wiley & Sons Ltd.
Figures




References
-
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL‐beta. Mol Cell. 2002;10(2):417‐426. - PubMed
-
- Delaleu N, Bickel M. Interleukin‐1 beta and interleukin‐18: regulation and activity in local inflammation. Periodontol 2000. 2004;35:42‐52. - PubMed
-
- Strowig T, Henao‐Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481(7381):278‐286. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1RR025005/NH/NIH HHS/United States
- KL2TR002490/TR/NCATS NIH HHS/United States
- HHSN268201100009I/HL/NHLBI NIH HHS/United States
- R01 DE011551/DE/NIDCR NIH HHS/United States
- P30 ES010126/ES/NIEHS NIH HHS/United States
- P30ES010126/ES/NIEHS NIH HHS/United States
- R01DE021418/DE/NIDCR NIH HHS/United States
- HHSN268201100010C/HL/NHLBI NIH HHS/United States
- UL1 RR025005/RR/NCRR NIH HHS/United States
- HHSN268201100008C/HL/NHLBI NIH HHS/United States
- HHSN268201100005G/HL/NHLBI NIH HHS/United States
- HHSN268201100008I/HL/NHLBI NIH HHS/United States
- K99DE027086/DE/NIDCR NIH HHS/United States
- R01 HL059367/HL/NHLBI NIH HHS/United States
- HHSN268201100007C/HL/NHLBI NIH HHS/United States
- R01DE11551/DE/NIDCR NIH HHS/United States
- R01HL59367/HL/NHLBI NIH HHS/United States
- HHSN268201100011I/HL/NHLBI NIH HHS/United States
- HHSN268201100011C/HL/NHLBI NIH HHS/United States
- R01 HL086694/HL/NHLBI NIH HHS/United States
- U01 HG004402/HG/NHGRI NIH HHS/United States
- U01HG004402/HG/NHGRI NIH HHS/United States
- K01DE027087/DE/NIDCR NIH HHS/United States
- HHSN268201100006C/HL/NHLBI NIH HHS/United States
- K99 DE027086/DE/NIDCR NIH HHS/United States
- K01 DE027087/DE/NIDCR NIH HHS/United States
- HHSN268201100005I/HL/NHLBI NIH HHS/United States
- R01HL087641/HL/NHLBI NIH HHS/United States
- HSN268201100012C/HL/NHLBI NIH HHS/United States
- KL2 TR002490/TR/NCATS NIH HHS/United States
- HHSN268201100009C/HL/NHLBI NIH HHS/United States
- HHSN268201100005C/HL/NHLBI NIH HHS/United States
- HHSN268201100007I/HL/NHLBI NIH HHS/United States
- R01 HL087641/HL/NHLBI NIH HHS/United States
- HHSN268200625226C/NH/NIH HHS/United States
- R01HL086694/HL/NHLBI NIH HHS/United States
- R01 DE021418/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources

