Role of muscle coactivation in adaptation of standing posture during arm reaching
- PMID: 31851559
- PMCID: PMC11960779
- DOI: 10.1152/jn.00939.2017
Role of muscle coactivation in adaptation of standing posture during arm reaching
Abstract
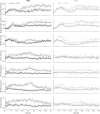
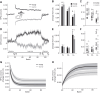
The ability to maintain stable, upright standing in the face of perturbations is a critical component of daily life. A common strategy for resisting perturbations and maintaining stability is muscle coactivation. Although arm muscle coactivation is often used during adaptation of seated reaching movements, little is known about postural muscle activation during concurrent adaptation of arm and standing posture to novel perturbations. In this study we investigate whether coactivation strategies are employed during adaptation of standing postural control, and how these strategies are prioritized for adaptation of standing posture and arm reaching, in two different postural stability conditions. Healthy adults practiced planar reaching movements while grasping the handle of a robotic arm and standing on a force plate; the robotic arm generated a velocity-dependent force field that created novel perturbations in the forward (more stable) or backward (less stable) direction. Surprisingly, the degree of arm and postural adaptation was not influenced by stability, with similar adaptation observed between conditions in the control of both arm movement and standing posture. We found that an early coactivation strategy can be used in postural adaptation, similar to what is observed in adaptation of arm reaching movements. However, the emergence of a coactivation strategy was dependent on perturbation direction. Despite similar adaptation in both directions, postural coactivation was largely specific to forward perturbations. Backward perturbations led to less coactivation and less modulation of postural muscle activity. These findings provide insight into how postural stability can affect prioritization of postural control objectives and movement adaptation strategies.NEW & NOTEWORTHY Muscle coactivation is a key strategy for modulating movement stability; this is centrally important in the control of standing posture. Our study investigates the little-known role of coactivation in adaptation of whole body standing postural control. We demonstrate that an early coactivation strategy can be used in postural adaptation, but muscle activation strategies may differ depending on postural stability conditions.
Keywords: anticipatory postural adjustment; coactivation; motor adaptation; neuromechanics; postural base of support.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures








References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

