Development of a pseudovirus-based assay for measuring neutralizing antibodies against Coxsackievirus A10
- PMID: 31851566
- PMCID: PMC7482785
- DOI: 10.1080/21645515.2019.1691404
Development of a pseudovirus-based assay for measuring neutralizing antibodies against Coxsackievirus A10
Abstract
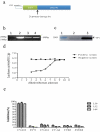
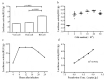
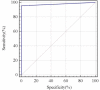
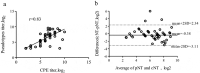
Coxsackievirus A10 (CV-A10) has recently emerged as a major pathogen of hand, foot, and mouth disease in children worldwide. Currently no effective treatments are available; development of anti-CV-A10 vaccine is a most cost-effective way for CV-A10 prevention. Robust assay to measure neutralizing antibody (NtAb) titres elicited by vaccination would greatly prompt anti-CV-A10 vaccine development. Compare to the traditional neutralization assay based on inhibition of cytopathic effects (herein after referred to as cNT) which is time-consuming and labor-intensive, in this study we developed an efficient high-throughput neutralization antibody assay based on CV-A10 pseudoviruses (herein after referred to as pNT). In the pNT, anti-CV-A10 NtAb titre was negatively corresponded with the relative luminescent unit (RLU) produced by luciferase reporter gene incorporated in pseudovirus genome. As described in this study, the NtAb against CV-A10 could be detected within 10-16 h, anti- CV-A10 NtAb in 67 human serum samples were measured in parallel with pNT and cNT assays, a good correlation (r = 0.83,p < .0001) and good agreement(97%) were shown between cNT and pNT, indicating that the pNT provides a rapid and convenient procedure for measuring NtAb production against anti-CV-A10 NtAb measurement.
Keywords: Coxsackievirus A10; HFMD; cytopathic effect; neutralizing antibody; pseudovirus.
Figures
References
-
- Munivenkatappa A, Yadav PD, Nyayanit DA, Majumdar TD, Sangal L, Jain S, Sinha DP, Shrivastava A, Mourya DT.. Molecular diversity of Coxsackievirus A10 circulating in the southern and northern region of India [2009–17]. Infect Genet Evol. 2018;66:101–10. doi:10.1016/j.meegid.2018.09.004. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
