Isolation of endothelial cells, pericytes and astrocytes from mouse brain
- PMID: 31851695
- PMCID: PMC6919623
- DOI: 10.1371/journal.pone.0226302
Isolation of endothelial cells, pericytes and astrocytes from mouse brain
Abstract
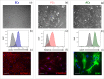
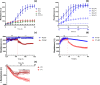
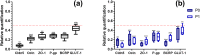
Primary cell isolation from the central nervous system (CNS) has allowed fundamental understanding of blood-brain barrier (BBB) properties. However, poorly described isolation techniques or suboptimal cellular purity has been a weak point of some published scientific articles. Here, we describe in detail how to isolate and enrich, using a common approach, endothelial cells (ECs) from adult mouse brains, as well as pericytes (PCs) and astrocytes (ACs) from newborn mouse brains. Our approach allowed the isolation of these three brain cell types with purities of around 90%. Furthermore, using our protocols, around 3 times more PCs and 2 times more ACs could be grown in culture, as compared to previously published protocols. The cells were identified and characterized using flow cytometry and confocal microscopy. The ability of ECs to form a tight monolayer was assessed for passages 0 to 3. The expression of claudin-5, occludin, zonula occludens-1, P-glycoprotein-1 and breast cancer resistance protein by ECs, as well as the ability of the cells to respond to cytokine stimuli (TNF-α, IFN-γ) was also investigated by q-PCR. The transcellular permeability of ECs was evaluated in the presence of pericytes or astrocytes in a Transwell® model by measuring the transendothelial electrical resistance (TEER), dextran-FITC and sodium fluorescein permeability. Overall, ECs at passages 0 and 1 featured the best properties valued in a BBB model. Furthermore, pericytes did not increase tightness of EC monolayers, whereas astrocytes did regardless of their seeding location. Finally, ECs resuspended in fetal bovine serum (FBS) and dimethyl sulfoxide (DMSO) could be cryopreserved in liquid nitrogen without affecting their phenotype nor their capacity to form a tight monolayer, thus allowing these primary cells to be used for various longitudinal in vitro studies of the blood-brain barrier.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
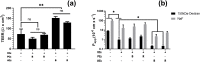

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

