Harnessing MerTK agonism for targeted therapeutics
- PMID: 31852344
- PMCID: PMC6927767
- DOI: 10.1080/19420862.2019.1685832
Harnessing MerTK agonism for targeted therapeutics
Abstract
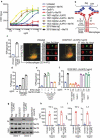
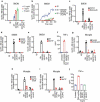
Phagocytosis plays important roles both in homeostasis and under pathological conditions. Fcγ receptor-mediated phagocytosis has been exploited as an integral mechanism for antibody-based therapies. Unlike Fcγ receptor-mediated phagocytosis, MerTK-mediated phagocytic clearance is immunologically silent. Here, we describe a bispecific antibody approach to harness MerTK for targeted clearance without inducing proinflammatory cytokine release associated with Fcγ receptor engagement. We generated bispecific antibodies targeting live B cells or amyloid beta aggregates to demonstrate the feasibility and versatility of this new approach.
Keywords: Fcγ receptor; MerTK; Phagocytosis; bispecific antibody; immunologically silent.
Figures
References
-
- Nimmerjahn F, Ravetch JV.. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–8. - PubMed
-
- Morkuniene R, Zvirbliene A, Dalgediene I, Cizas P, Jankeviciute S, Baliutyte G, Jokubka R, Jankunec M, Valincius G, Borutaite V, et al. Antibodies bound to Abeta oligomers potentiate the neurotoxicity of Abeta by activating microglia. J Neurochem. 2013;126:604–15. doi:10.1111/jnc.2013.126.issue-5. - DOI - PubMed
-
- Rinne JO, Brooks DJ, Rossor MN, Fox NC, Bullock R, Klunk WE, Mathis CA, Blennow K, Barakos J, Okello AA, et al. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer’s disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 2010;9:363–72. doi:10.1016/S1474-4422(10)70043-0. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
