Full-length transcriptome analysis of shade-induced promotion of tuber production in Pinellia ternata
- PMID: 31852442
- PMCID: PMC6921527
- DOI: 10.1186/s12870-019-2197-9
Full-length transcriptome analysis of shade-induced promotion of tuber production in Pinellia ternata
Abstract
Background: Pinellia ternata is native to China and has been used as a traditional herb due to its antiemetic, antitussive, analgesic, and anxiolytic effects. When exposed to strong light intensity and high temperature during the reproductive growth process, P. ternata withers in a phenomenon known as "sprout tumble", which largely limits tuber production. Shade was previously found to delay sprout tumble formation (STF); however, no information exists regarding this process at the molecular level. Hence, we determined the genes involved in tuber development and STF in P. ternata.
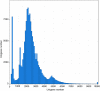
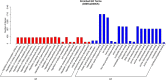
Results: Compared to that with natural sun-light (control), shade significantly induced chlorophyll accumulation, increased chlorophyll fluorescence parameters including initial fluorescence, maximal fluorescence, and qP, and dramatically repressed chlorophyll a:b and NPQ. Catalase (CAT) activity was largely induced by shade, and tuber products were largely increased in this environment. Transcriptome profiles of P. ternata grown in natural sun-light and shaded environments were analyzed by a combination of next generation sequencing (NGS) and third generation single-molecule real-time (SMRT) sequencing. Corrections of SMRT long reads based on NGS short reads yielded 136,163 non-redundant transcripts, with an average N50 length of 2578 bp. In total, 6738 deferentially-expressed genes (DEGs) were obtained from the comparisons, specifically D5S vs D5CK, D20S vs D20CK, D20S vs D5S, and D20CK vs D5CK, of which, 6384 DEGs (94.8%) were generated from the D20S vs D20CK comparison. Gene annotation and functional analyses revealed that these genes were related to auxin signal transduction, polysaccharide and sugar metabolism, phenylpropanoid biosynthesis, and photosynthesis. Moreover, the expression of genes enriched in photosynthesis appeared to be significantly altered by shade. The expression patterns of 16 candidate genes were consistent with changes in their transcript abundance as identified by RNA-Seq, and these might contribute to STF and tuber production.
Conclusion: The full-length transcripts identified in this study have provided a more accurate depiction of P. ternata gene transcription. Further, we identified potential genes involved in STF and tuber growth. Such data could serve as a genetic resource and a foundation for further research on this important traditional herb.
Keywords: Pinellia ternata; Shade; Single-molecule real-time sequencing; Sprout tumble; Transcriptome; Tuber production.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Zhang JY, Guo QS, Zheng DS. Genetic diversity analysis of Pinellia ternata based on SRAP and TRAP markers. Biochem Syst Ecol. 2013;50:258–265. doi: 10.1016/j.bse.2013.03.052. - DOI
-
- Moon BC, Kim WJ, Ji Y, Lee YM, Kang YM, Choi G. Molecular identification of the traditional herbal medicines, Arisaematis Rhizoma and Pinelliae Tuber, and common adulterants via universal DNA barcode sequences. Genet Mol Res 2016. 15(1):gmr.15017064. - PubMed
-
- Mao ZC, Peng ZS. Progress on research of rapid propagation system of Pinellia ternata. China j Chinese materia medica. 2003;28(3):193–195. - PubMed
-
- Zeng XQ, Peng ZS. Growth and propagation of wild Pinellia ternata in cultivation. China j Chinese materia medica. 2008;33(8):878–883. - PubMed
MeSH terms
Grants and funding
- 81803665/National Natural Science Foundation of China
- 81573518/National Natural Science Foundation of China
- 31501368/National Natural Science Foundation of China
- 1608085MC52/Natural Science Foundation of Anhui Province
- KJ2018A0403/University Natural Science Research Project of Anhui Province
- KJ2015ZD35/University Natural Science Research Project of Anhui Province
- KJ2018B15/University Natural Science Research Project of Anhui Province
- KJ2017A846/University Natural Science Research Project of Anhui Province
- KJ2016A644/University Natural Science Research Project of Anhui Province
- KJ2019B07/University Natural Science Research Project of Anhui Province
- KJ2015TD001/the Innovation Team of Scientific Research Platform of Anhui Province
LinkOut - more resources
Full Text Sources
Miscellaneous

