Chemical composition of food induces plasticity in digestive morphology in larvae of Rana temporaria
- PMID: 31852656
- PMCID: PMC6955212
- DOI: 10.1242/bio.048041
Chemical composition of food induces plasticity in digestive morphology in larvae of Rana temporaria
Abstract
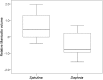
Food conditions are changing due to anthropogenic activities and natural sources and thus, many species are exposed to new challenges. Animals might cope with altered quantitative and qualitative composition [i.e. variable protein, nitrogen (N) and energy content] of food by exhibiting trophic and digestive plasticity. We examined experimentally whether tadpoles of the common frog (Rana temporaria) exhibit phenotypic plasticity of the oral apparatus and intestinal morphology when raised on a diet of either low (i.e. Spirulina algae) or high protein, N and energy content (i.e. Daphnia pulex). Whereas intestinal morphology was highly plastic, oral morphology did not respond plastically to different chemical compositions of food. Tadpoles that were fed food with low protein and N content and low-energy density developed significantly longer guts and a larger larval stomachs than tadpoles raised on high protein, N and an energetically dense diet, and developed a different intestinal surface morphology. Body sizes of the treatment groups were similar, indicating that tadpoles fully compensated for low protein, N and energy diet by developing longer intestines. The ability of a species, R. temporaria, to respond plastically to environmental variation indicates that this species might have the potential to cope with new conditions during climate change.
Keywords: Adaptability; Climate change; Gut length; Nutrient content; Oral papillae; Protein content.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures



References
-
- Altig R. and Johnston G. F. (1989). Guilds of anuran larvae: relationships among developmental modes, morphologies, and habitats. Herpetol. Monogr. 3, 81 10.2307/1466987 - DOI
-
- Babak E. (1905). Über die morphogenetische Reaktion des Darmkanals der Froschlarve auf Muskelproteine verschiedener Tierklassen. Beitrage zur Chemischen Physiologie und Pathologie 7, 323-330.
-
- Berven K. A. (1990). Factors affecting population fluctuations in larval and adult stages of the wood frog (Rana Sylvatica). Ecology 71, 1599-1608. 10.2307/1938295 - DOI
-
- Berven K. A. and Gill D. E. (1983). Interpreting geographic variation in life-history traits. Am. Zool. 23, 85-97. 10.1093/icb/23.1.85 - DOI
LinkOut - more resources
Full Text Sources

