Impact of a Rapid Blood Culture Diagnostic Test in a Children's Hospital Depends on Gram-Positive versus Gram-Negative Organism and Day versus Night Shift
- PMID: 31852761
- PMCID: PMC7098773
- DOI: 10.1128/JCM.01400-19
Impact of a Rapid Blood Culture Diagnostic Test in a Children's Hospital Depends on Gram-Positive versus Gram-Negative Organism and Day versus Night Shift
Abstract
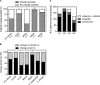
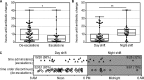
Rapid diagnostic tests (RDTs) for bloodstream infections (BSIs) decrease the time to organism identification and resistance detection. RDTs are associated with early deescalation of therapy for Gram-positive BSIs. However, it is less clear how RDTs influence antibiotic management for Gram-negative BSIs and whether RDT results are acted on during off-hours. We performed a single-center, retrospective review of children with BSI and Verigene (VG) testing at a children's hospital. Of the 301 positive cultures included in the study (196 Gram-positive, 44 Gram-negative, 32 polymicrobial, and 29 non-VG targets), the VG result had potential to impact antibiotic selection in 171 cases; among these, antibiotic changes occurred in 119 (70%) cases. For Gram-negative cultures, the Verigene result correlated with unnecessary antibiotic escalation and exposure to broader-spectrum antibiotics than needed. In contrast, for Gram-positive cultures, the VG results correlated with appropriate antibiotic selection. VG results permitted early deescalation for methicillin-susceptible Staphylococcus aureus (MSSA) (19/24 [79%]) and avoidance of antibiotics for skin contaminants (30/85 [35%]). Antibiotic changes occurred more quickly during the day than at night (4.6 versus 11.7 h, respectively; P < 0.05), and antibiotic escalations occurred more quickly than did deescalations (4.1 versus 10.1 h, P < 0.01). In a pediatric institution with a low prevalence of Gram-negative resistance, the VG RDT facilitated antibiotic optimization for Gram-positive BSIs but led to unnecessary escalation of antibiotics for Gram-negative BSIs. The time to action was slower for RDT results reported at night than during the day. Laboratories should consider these factors when implementing blood culture RDTs.
Keywords: antibiotic stewardship; bacteremia; pediatric; pediatric infectious diseases; rapid diagnostic tests.
Copyright © 2020 American Society for Microbiology.
Figures


Comment in
-
Use of Rapid Diagnostics To Manage Pediatric Bloodstream Infections? You Bet Your ASP!J Clin Microbiol. 2020 Mar 25;58(4):e02082-19. doi: 10.1128/JCM.02082-19. Print 2020 Mar 25. J Clin Microbiol. 2020. PMID: 31969424 Free PMC article.
Similar articles
-
Predictive Value of Direct Disk Diffusion Testing from Positive Blood Cultures in a Children's Hospital and Its Utility in Antimicrobial Stewardship.J Clin Microbiol. 2021 May 19;59(6):e02445-20. doi: 10.1128/JCM.02445-20. Print 2021 May 19. J Clin Microbiol. 2021. PMID: 33692138 Free PMC article.
-
Randomized Trial Evaluating Clinical Impact of RAPid IDentification and Susceptibility Testing for Gram-negative Bacteremia: RAPIDS-GN.Clin Infect Dis. 2021 Jul 1;73(1):e39-e46. doi: 10.1093/cid/ciaa528. Clin Infect Dis. 2021. PMID: 32374822 Free PMC article. Clinical Trial.
-
Clinical Performance and Impact of Accelerate Pheno for Gram-negative Bacteremia in Hospitalized Children.Clin Ther. 2020 Sep;42(9):1630-1636. doi: 10.1016/j.clinthera.2020.07.015. Epub 2020 Aug 18. Clin Ther. 2020. PMID: 32826063
-
Rapid microbiological tests for bloodstream infections due to multidrug resistant Gram-negative bacteria: therapeutic implications.Clin Microbiol Infect. 2020 Jun;26(6):713-722. doi: 10.1016/j.cmi.2019.09.023. Epub 2019 Oct 11. Clin Microbiol Infect. 2020. PMID: 31610299 Review.
-
Interplay between Rapid Diagnostic Tests and Antimicrobial Stewardship Programs among Patients with Bloodstream and Other Severe Infections.J Appl Lab Med. 2019 Jan;3(4):601-616. doi: 10.1373/jalm.2018.026450. Epub 2018 Nov 20. J Appl Lab Med. 2019. PMID: 31639729 Review.
Cited by
-
Predictive Value of Direct Disk Diffusion Testing from Positive Blood Cultures in a Children's Hospital and Its Utility in Antimicrobial Stewardship.J Clin Microbiol. 2021 May 19;59(6):e02445-20. doi: 10.1128/JCM.02445-20. Print 2021 May 19. J Clin Microbiol. 2021. PMID: 33692138 Free PMC article.
-
Rapid Direct Identification of Microbial Pathogens and Antimicrobial Resistance Genes in Positive Blood Cultures Using a Fully Automated Multiplex PCR Assay.J Korean Med Sci. 2024 May 6;39(17):e157. doi: 10.3346/jkms.2024.39.e157. J Korean Med Sci. 2024. PMID: 38711319 Free PMC article.
-
Use of Rapid Diagnostics To Manage Pediatric Bloodstream Infections? You Bet Your ASP!J Clin Microbiol. 2020 Mar 25;58(4):e02082-19. doi: 10.1128/JCM.02082-19. Print 2020 Mar 25. J Clin Microbiol. 2020. PMID: 31969424 Free PMC article.
-
Modern Blood Culture: Management Decisions and Method Options.Clin Lab Med. 2020 Dec;40(4):379-392. doi: 10.1016/j.cll.2020.07.001. Epub 2020 Sep 19. Clin Lab Med. 2020. PMID: 33121610 Free PMC article. Review.
-
Artificial Intelligence model to predict resistances in Gram-negative bloodstream infections.NPJ Digit Med. 2025 May 29;8(1):319. doi: 10.1038/s41746-025-01696-x. NPJ Digit Med. 2025. PMID: 40442363 Free PMC article.
References
-
- Banerjee R, Teng CB, Cunningham SA, Ihde SM, Steckelberg JM, Moriarty JP, Shah ND, Mandrekar JN, Patel R. 2015. Randomized trial of rapid multiplex polymerase chain reaction-based blood culture identification and susceptibility testing. Clin Infect Dis 61:1071–1080. doi:10.1093/cid/civ447. - DOI - PMC - PubMed
-
- Box MJ, Sullivan EL, Ortwine KN, Parmenter MA, Quigley MM, Aguilar-Higgins LM, MacIntosh CL, Goerke KF, Lim RA. 2015. Outcomes of rapid identification for gram-positive bacteremia in combination with antibiotic stewardship at a community-based hospital system. Pharmacotherapy 35:269–276. doi:10.1002/phar.1557. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

