Optimizing DNA Extraction Methods for Nanopore Sequencing of Neisseria gonorrhoeae Directly from Urine Samples
- PMID: 31852766
- PMCID: PMC7041563
- DOI: 10.1128/JCM.01822-19
Optimizing DNA Extraction Methods for Nanopore Sequencing of Neisseria gonorrhoeae Directly from Urine Samples
Abstract
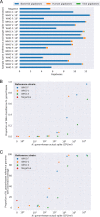
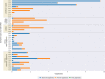
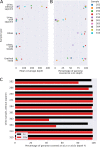
Empirical gonorrhea treatment at initial diagnosis reduces onward transmission. However, increasing resistance to multiple antibiotics may necessitate waiting for culture-based diagnostics to select an effective treatment. There is a need for same-day culture-free diagnostics that identify infection and detect antimicrobial resistance. We investigated if Nanopore sequencing can detect sufficient Neisseria gonorrhoeae DNA to reconstruct whole genomes directly from urine samples. We used N. gonorrhoeae-spiked urine samples and samples from gonorrhea infections to determine optimal DNA extraction methods that maximize the amount of N. gonorrhoeae DNA sequenced while minimizing contaminating host DNA. In simulated infections, the Qiagen UCP pathogen mini kit provided the highest ratio of N. gonorrhoeae to human DNA and the most consistent results. Depletion of human DNA with saponin increased N. gonorrhoeae yields in simulated infections but decreased yields in clinical samples. In 10 urine samples from men with symptomatic urethral gonorrhea, ≥92.8% coverage of an N. gonorrhoeae reference genome was achieved in all samples, with ≥93.8% coverage breath at ≥10-fold depth in 7 (70%) samples. In simulated infections, if ≥104 CFU/ml of N. gonorrhoeae was present, sequencing of the large majority of the genome was frequently achieved. N. gonorrhoeae could also be detected from urine in cobas PCR medium tubes and from urethral swabs and in the presence of simulated Chlamydia coinfection. Using Nanopore sequencing of urine samples from men with urethral gonorrhea, sufficient data can be obtained to reconstruct whole genomes in the majority of samples without the need for culture.
Keywords: DNA extraction; Nanopore sequencing; Neisseria gonorrhoeae; whole-genome sequencing.
Copyright © 2020 Street et al.
Figures


References
-
- Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, Stevens G, Gottlieb S, Kiarie J, Temmerman M. 2015. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 10:e0143304. doi:10.1371/journal.pone.0143304. - DOI - PMC - PubMed
-
- Fifer H, Saunders J, Soni S, Sadiq T, Fitzgerald M. 2019. British Association for Sexual Health and HIV national guideline for the management of infection with Neisseria gonorrhoeae. British Association for Sexual Health and HIV, Bacclesfield, England: https://www.bashhguidelines.org/media/1208/gc-2019.pdf. - PubMed
-
- Eyre DW, Town K, Street T, Barker L, Sanderson N, Cole MJ, Mohammed H, Pitt R, Gobin M, Irish C, Gardiner D, Sedgwick J, Beck C, Saunders J, Turbitt D, Cook C, Phin N, Nathan B, Horner P, Fifer H. 2019. Detection in the United Kingdom of the Neisseria gonorrhoeae FC428 clone, with ceftriaxone resistance and intermediate resistance to azithromycin, October to December 2018. Eurosurveillance 24:1900147. doi:10.2807/1560-7917.ES.2019.24.10.1900147. - DOI - PMC - PubMed
-
- Eyre DW, Sanderson ND, Lord E, Regisford-Reimmer N, Chau K, Barker L, Morgan M, Newnham R, Golparian D, Unemo M, Crook DW, Peto TE, Hughes G, Cole MJ, Fifer H, Edwards A, Andersson MI. 2018. Gonorrhoea treatment failure caused by a Neisseria gonorrhoeae strain with combined ceftriaxone and high-level azithromycin resistance, England, February 2018. Eurosurveillance 23:364. doi:10.2807/1560-7917.ES.2018.23.27.1800323. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

