Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study
- PMID: 31852769
- PMCID: PMC7229896
- DOI: 10.1136/gutjnl-2019-319630
Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study
Abstract
Objective: Faecal microbiota transplantation (FMT) from healthy donors to patients with irritable bowel syndrome (IBS) has been attempted in two previous double-blind, placebo-controlled studies. While one of those studies found improvement of the IBS symptoms, the other found no effect. The present study was conducted to clarify these contradictory findings.
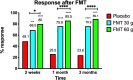
Design: This randomised, double-blind, placebo-controlled study randomised 165 patients with IBS to placebo (own faeces), 30 g FMT or 60 g FMT at a ratio of 1:1:1. The material for FMT was obtained from one healthy, well-characterised donor, frozen and administered via gastroscope. The primary outcome was a reduction in the IBS symptoms at 3 months after FMT (response). A response was defined as a decrease of 50 or more points in the total IBS symptom score. The secondary outcome was a reduction in the dysbiosis index (DI) and a change in the intestinal bacterial profile, analysed by 16S rRNA gene sequencing, at 1 month following FMT.
Results: Responses occurred in 23.6%, 76.9% (p<0.0001) and 89.1% (p<00.0001) of the patients who received placebo, 30 g FMT and 60 g FMT, respectively. These were accompanied by significant improvements in fatigue and the quality of life in patients who received FMT. The intestinal bacterial profiles changed also significantly in the groups received FMT. The FMT adverse events were mild self-limiting gastrointestinal symptoms.
Conclusions: FMT is an effective treatment for patients with IBS. Utilising a well-defined donor with a normal DI and favourable specific microbial signature is essential for successful FMT. The response to FMT increases with the dose. Trial registration www.clinicaltrials.gov (NCT03822299) and www.cristin.no (ID657402).
Keywords: colonic microflora; irritable bowel syndrome; lactobacillus.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures



Comment in
-
Faecal microbial transplantation in IBS: ready for prime time?Gut. 2020 May;69(5):795-796. doi: 10.1136/gutjnl-2019-320411. Epub 2020 Feb 26. Gut. 2020. PMID: 32102925 No abstract available.
-
Faecal microbiota transplantation in IBS - new evidence for success?Nat Rev Gastroenterol Hepatol. 2020 Apr;17(4):199-200. doi: 10.1038/s41575-020-0282-z. Nat Rev Gastroenterol Hepatol. 2020. PMID: 32107473 No abstract available.
-
FMT in IBS: a call for caution.Gut. 2021 Feb;70(2):431. doi: 10.1136/gutjnl-2020-321529. Epub 2020 May 15. Gut. 2021. PMID: 32414814 No abstract available.
-
In irritable bowel syndrome, fecal microbiota transplantation improved symptoms at 3 months.Ann Intern Med. 2020 May 19;172(10):JC52. doi: 10.7326/ACPJ202005190-052. Ann Intern Med. 2020. PMID: 32422097
-
FMT in IBS: how cautious should we be?Gut. 2021 Mar;70(3):626-628. doi: 10.1136/gutjnl-2020-322038. Epub 2020 Jul 14. Gut. 2021. PMID: 32665338 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
