An Open-Label, Single-Arm, Two-Stage, Multicenter, Phase II Study to Evaluate the Efficacy of TLC388 and Genomic Analysis for Poorly Differentiated Neuroendocrine Carcinomas
- PMID: 31852810
- PMCID: PMC7216444
- DOI: 10.1634/theoncologist.2019-0490
An Open-Label, Single-Arm, Two-Stage, Multicenter, Phase II Study to Evaluate the Efficacy of TLC388 and Genomic Analysis for Poorly Differentiated Neuroendocrine Carcinomas
Abstract
Background: The discovery of effective therapeutic options for treating metastatic poorly differentiated neuroendocrine carcinoma (NEC) after prior platinum-based chemotherapy remains elusive. This study analyzed the efficacy of TLC388 (Lipotecan) Hydrochloride, a novel camptothecin analog, for pretreated patients with metastatic NEC.
Methods: This single-arm, two-stage, phase II clinical trial was conducted at four community and academic centers in Taiwan. Patients aged 20 years or older with confirmed metastatic NEC and who had received prior systemic therapy with etoposide plus cisplatin were enrolled between July 2015 and May 2018. Patients received 40 mg/m2 of TLC388 intravenously on days 1, 8, and 15 of a 28-day cycle until disease progression or unacceptable toxic effects. Gene mutations were analyzed by next-generation sequencing.
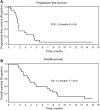
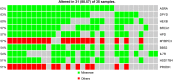
Results: Twenty-three patients with a median age of 61 (range, 44-73) years, 18 of whom were men (78%), were enrolled. Patients received a median of 2 (range, 0-6) treatment cycles. Among 20 evaluable patients, 3 patients exhibited stable disease and no patient experienced a complete or partial remission, resulting in a disease control rate of 15%. Median progression-free survival was 1.8 (95% confidence interval [CI], 0.4-15) months, and the median overall survival was 4.3 (95% CI, 1.7-15) months. The most common treatment-related hematologic adverse events at grade 3 or higher were leukopenia (22.7%), anemia (31.8%), and thrombocytopenia (18.2%). The most frequent mutated genes in 35 patients with NEC were ARSA, DPYD, HEXB, BRCA1, HPD, MYBPC3, BBS2, IL7R, HSD17B4, and PRODH.
Conclusion: TLC388 demonstrates limited antitumor activity in metastatic NEC. ClinicalTrials.gov identifier: NCT02457273.
Implications for practice: Poorly differentiated neuroendocrine carcinomas (NECs) are rare and aggressive. Currently, effective therapeutic options for treating metastatic poorly differentiated NECs beyond platinum-based chemotherapy remain elusive. In this single-arm, multicenter, phase II study, 23 patients with NEC were enrolled and received TLC388 (Lipotecan) Hydrochloride, which is a novel camptothecin analog. The results demonstrated the disease control rate of 15%, the median progression-free survival of 1.8 (95% confidence interval [CI], 0.4-15) months, and the median overall survival of 4.3 (95% CI, 1.7-15) months. Most importantly, several novel genetic mutations and pathways were identified. These results offer the opportunity to develop future treatment strategies in this rare cancer.
Keywords: Genetic profiles; Neuroendocrine carcinoma; TLC-388; Topotecan.
© AlphaMed Press 2019.
Conflict of interest statement
Figures
Similar articles
-
A randomized phase II trial of Captem or Folfiri as second-line therapy in neuroendocrine carcinomas.Eur J Cancer. 2024 Sep;208:114129. doi: 10.1016/j.ejca.2024.114129. Epub 2024 May 25. Eur J Cancer. 2024. PMID: 39002347 Clinical Trial.
-
Efficacy of topotecan in pretreated metastatic poorly differentiated extrapulmonary neuroendocrine carcinoma.Cancer Med. 2016 Sep;5(9):2261-7. doi: 10.1002/cam4.807. Epub 2016 Jul 25. Cancer Med. 2016. PMID: 27456539 Free PMC article.
-
Capecitabine, Oxaliplatin, Irinotecan, and Bevacizumab Combination Followed by Pazopanib Plus Capecitabine Maintenance for High-Grade Gastrointestinal Neuroendocrine Carcinomas.Am J Clin Oncol. 2020 May;43(5):305-310. doi: 10.1097/COC.0000000000000668. Am J Clin Oncol. 2020. PMID: 32343515 Clinical Trial.
-
Neuroendocrine Cancer, Therapeutic Strategies in G3 Cancers.Digestion. 2017;95(2):109-114. doi: 10.1159/000454761. Epub 2017 Feb 4. Digestion. 2017. PMID: 28161703 Review.
-
Chemotherapy for advanced poorly differentiated pancreatic neuroendocrine carcinoma.J Hepatobiliary Pancreat Sci. 2015 Aug;22(8):623-7. doi: 10.1002/jhbp.228. Epub 2015 Mar 5. J Hepatobiliary Pancreat Sci. 2015. PMID: 25755102 Review.
Cited by
-
Selection of Chemotherapy in Advanced Poorly Differentiated Extra-Pulmonary Neuroendocrine Carcinoma.Cancers (Basel). 2023 Oct 11;15(20):4951. doi: 10.3390/cancers15204951. Cancers (Basel). 2023. PMID: 37894318 Free PMC article. Review.
-
Inefficiency of two-stage designs in phase II oncology clinical trials with high proportion of inevaluable patients.Contemp Clin Trials. 2022 Sep;120:106849. doi: 10.1016/j.cct.2022.106849. Epub 2022 Jul 19. Contemp Clin Trials. 2022. PMID: 35868503 Free PMC article. Clinical Trial.
-
Systemic Treatment of Gastroenteropancreatic Neuroendocrine Carcinoma.Curr Treat Options Oncol. 2021 Jun 10;22(8):68. doi: 10.1007/s11864-021-00866-9. Curr Treat Options Oncol. 2021. PMID: 34110508 Free PMC article. Review.
-
Second-line treatment in patients with advanced extra-pulmonary poorly differentiated neuroendocrine carcinoma: a systematic review and meta-analysis.Ther Adv Med Oncol. 2020 Apr 27;12:1758835920915299. doi: 10.1177/1758835920915299. eCollection 2020. Ther Adv Med Oncol. 2020. PMID: 32426044 Free PMC article. Review.
-
Comparative Outcomes of Second-line Topoisomerase-I Inhibitor Therapies on Neuroendocrine Carcinoma.J Gastrointest Cancer. 2023 Mar;54(1):73-79. doi: 10.1007/s12029-021-00800-0. Epub 2022 Jan 10. J Gastrointest Cancer. 2023. PMID: 35006522 Free PMC article.
References
-
- Ahlman H, Nilsson O, McNicol AM et al. Poorly‐differentiated endocrine carcinomas of midgut and hindgut origin. Neuroendocrinology 2008;87:40–46. - PubMed
-
- Nilsson O, Van Cutsem E, Delle Fave G et al. Poorly differentiated carcinomas of the foregut (gastric, duodenal and pancreatic). Neuroendocrinology 2006;84:212–215. - PubMed
-
- Majhail NS, Elson P, Bukowski RM. Therapy and outcome of small cell carcinoma of the kidney: Report of two cases and a systematic review of the literature. Cancer 2003;97:1436‐1441. - PubMed
-
- Mills SE, Cooper PH, Garland TA et al. Small cell undifferentiated carcinoma of the larynx. Report of two patients and review of 13 additional cases. Cancer 1983;51:116–120. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

