Human muscle-derived CLEC14A-positive cells regenerate muscle independent of PAX7
- PMID: 31852888
- PMCID: PMC6920394
- DOI: 10.1038/s41467-019-13650-z
Human muscle-derived CLEC14A-positive cells regenerate muscle independent of PAX7
Abstract
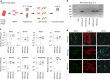
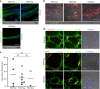
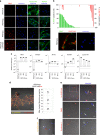
Skeletal muscle stem cells, called satellite cells and defined by the transcription factor PAX7, are responsible for postnatal muscle growth, homeostasis and regeneration. Attempts to utilize the regenerative potential of muscle stem cells for therapeutic purposes so far failed. We previously established the existence of human PAX7-positive cell colonies with high regenerative potential. We now identified PAX7-negative human muscle-derived cell colonies also positive for the myogenic markers desmin and MYF5. These include cells from a patient with a homozygous PAX7 c.86-1G > A mutation (PAX7null). Single cell and bulk transcriptome analysis show high intra- and inter-donor heterogeneity and reveal the endothelial cell marker CLEC14A to be highly expressed in PAX7null cells. All PAX7-negative cell populations, including PAX7null, form myofibers after transplantation into mice, and regenerate muscle after reinjury. Transplanted PAX7neg cells repopulate the satellite cell niche where they re-express PAX7, or, strikingly, CLEC14A. In conclusion, transplanted human cells do not depend on PAX7 for muscle regeneration.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

