CETSA-based target engagement of taxanes as biomarkers for efficacy and resistance
- PMID: 31852908
- PMCID: PMC6920357
- DOI: 10.1038/s41598-019-55526-8
CETSA-based target engagement of taxanes as biomarkers for efficacy and resistance
Abstract
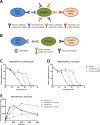
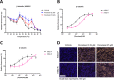
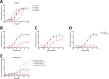
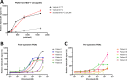
The use of taxanes has for decades been crucial for treatment of several cancers. A major limitation of these therapies is inherent or acquired drug resistance. A key to improved outcome of taxane-based therapies is to develop tools to predict and monitor drug efficacy and resistance in the clinical setting allowing for treatment and dose stratification for individual patients. To assess treatment efficacy up to the level of drug target engagement, we have established several formats of tubulin-specific Cellular Thermal Shift Assays (CETSAs). This technique was evaluated in breast and prostate cancer models and in a cohort of breast cancer patients. Here we show that taxanes induce significant CETSA shifts in cell lines as well as in animal models including patient-derived xenograft (PDX) models. Furthermore, isothermal dose response CETSA measurements allowed for drugs to be rapidly ranked according to their reported potency. Using multidrug resistant cancer cell lines and taxane-resistant PDX models we demonstrate that CETSA can identify taxane resistance up to the level of target engagement. An imaging-based CETSA format was also established, which in principle allows for taxane target engagement to be accessed in specific cell types in complex cell mixtures. Using a highly sensitive implementation of CETSA, we measured target engagement in fine needle aspirates from breast cancer patients, revealing a range of different sensitivities. Together, our data support that CETSA is a robust tool for assessing taxane target engagement in preclinical models and clinical material and therefore should be evaluated as a prognostic tool during taxane-based therapies.
Conflict of interest statement
PN is the inventor of CETSA, holder of patents related to the CETSA method, and the co-founder of Pelago Bioscience AB. All the other authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

