Tumor organoid-T-cell coculture systems
- PMID: 31853056
- PMCID: PMC7610702
- DOI: 10.1038/s41596-019-0232-9
Tumor organoid-T-cell coculture systems
Abstract
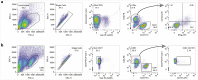
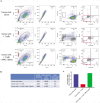
T cells are key players in cancer immunotherapy, but strategies to expand tumor-reactive cells and study their interactions with tumor cells at the level of an individual patient are limited. Here we describe the generation and functional assessment of tumor-reactive T cells based on cocultures of tumor organoids and autologous peripheral blood lymphocytes. The procedure consists of an initial coculture of 2 weeks, in which tumor-reactive T cells are first expanded in the presence of (IFNγ-stimulated) autologous tumor cells. Subsequently, T cells are evaluated for their capacity to carry out effector functions (IFNγ secretion and degranulation) after recognition of tumor cells, and their capacity to kill tumor organoids. This strategy is unique in its use of peripheral blood as a source of tumor-reactive T cells in an antigen-agnostic manner. In 2 weeks, tumor-reactive CD8+ T-cell populations can be obtained from ~33-50% of samples from patients with non-small-cell lung cancer (NSCLC) and microsatellite-instable colorectal cancer (CRC). This enables the establishment of ex vivo test systems for T-cell-based immunotherapy at the level of the individual patient.
Conflict of interest statement
There are no competing financial interests to report.
Figures



References
-
- Blank CU, Haanen JB, Ribas A, Schumacher TN. The “cancer immunogram”. Science. 2016;352:658–660. - PubMed
-
- Dijkstra KK, Voabil P, Schumacher TN, Voest EE. Genomics- and transcriptomics-based patient selection for cancer treatment with immune checkpoint inhibitors: a review. JAMA Oncol. 2016;2:1490–1495. - PubMed
-
- Pitt JM, et al. Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and –extrinsic factors. Immunity. 2016;44:1255–1269. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

